Reports: UNI553906-UNI5: Surface Selective Laser Spectroscopy Investigation of Photochemistry of Aromatic Compounds at the Air-Water and Oil-Water Interface
Mahamud Subir, PhD, Ball State University
Using the support from the ACS Petroleum Research Fund, my students and I have made substantial progress toward our goal to investigate surface affinity and photochemistry of aromatic compounds at the air-water interface.
1. Building and Optimizing SHG Experimental Setup
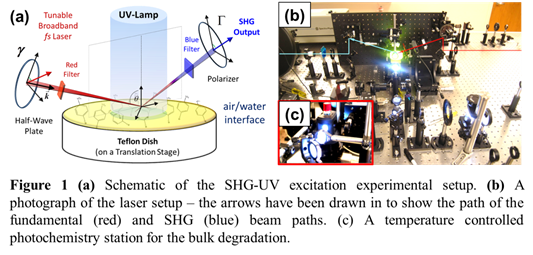
We have constructed an experimental setup (a schematic diagram is shown in Fig. 1a) that combines the capabilities of monitoring surface adsorption of aromatic organic compounds and investigate photo-degradation upon exposure to UV radiation using second harmonic generation (SHG) spectroscopy. Briefly, a broadband, tunable near IR beam (~70 fs) is focused onto the sample to generate SHG and a collimated UV light from a Xenon lamp is directed onto the sample (Fig. 1b). An additional photochemistry station has also been installed, which includes a temperature controlled cell holder to carry out photochemical degradation in the bulk solution (Fig. 1c). Generation of electronic sum frequency (ESFG) has also been attempted; however, weak efficiency of the frequency doubling crystal precluded generation of strong ESFG signal from samples exhibiting electronic transitions below 350 nm. Thus, our primary focus during this period has been to utilize SHG to study molecules with absorption band in the range of 350nm – 500nm. Major equipment used in this project was obtained using Ball State University start-up fund.
2. Adsorption of Aromatic Compounds at the Air/Water Interface
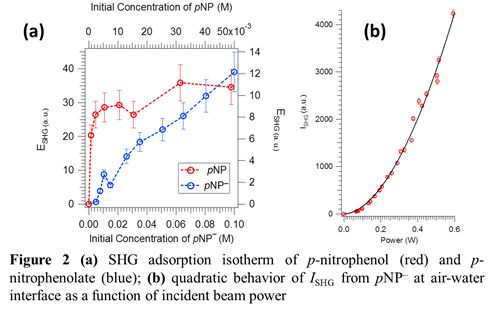
Adsorption of p-nitrophenol (pNP; pH = 2) and p-nitrophenolate (pNP–; pH = 13) to the air-aqueous interface have been studied using optical SHG spectroscopy. The SHG adsorption isotherms at different combination of polarizations have been collected. Selected pNP and pNP–isotherms are shown in figure 2a. The data demonstrates that there is an increase in ESHG as a function of bulk concentrations of the compounds, indicating increased surface population of these aromatic compounds at the air water interface. It is evident that pNP has greater affinity for the air-water interface than pNP–. We have further verified that this signal is indeed SHG by performing a power dependence study. The quadratic increase in the SHG intensity from pNP– as a function of incident beam power (Fig. 2b) shows that the generated signal at 2w corresponds to a second order process. Understanding the adsorption process of these aromatic compounds is critical for further photochemical studies at the air-water interface.
3. Systematic Investigation of Surface and Bulk Photochemistry
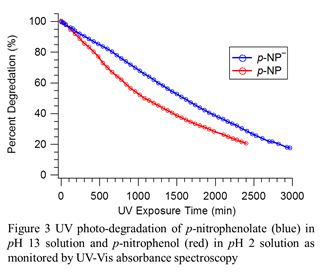
With the adsorption of pNP– at the air-water interface verified (fig. 2) we have carried out its photo-degradation in the bulk solution and at the surface. The kinetics of bulk photo-degradation upon UV radiation is shown in figure 3. The percent degradation is based on the change in peak absorbance upon irradiation. The pKa of pNP is 7; thus, the use of pH solutions of 2 and 13 ensured systematic photo-degradation of two distinct species, pNP and pNP–, respectively. The photo-degradation of pNP– at the air-aqueous interface was studied using the experimental setup described above. Comparison of surface photochemistry of pNP– with that of the bulk photo-degradation revealed a significant difference at the surface. However, our results show that the surface photo-degradation is complicated by many factors including, but not limited to, depletion of the reactant species, adsorption/desorption of newly formed products, and molecular orientation. Our current effort involves systematic investigation of these factors and exploring the effect of air-hexane interface.
4. Exploration of Additional Aromatic Compounds
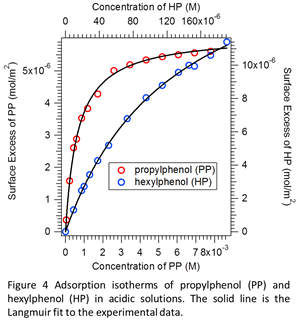
While our exploration with surface photochemistry is ongoing, we are also focused on identifying and characterizing different aromatic molecules to be studied using SHG surface photochemistry setup. Using surface tensiometry (ST), we established Gibbs adsorption isotherms of various long chain phenols at the air-water interface. Figure 4 depicts surface tension measurements of selected compounds we have studied systematically under controlled pH. Students in my group have also utilized a Langmuir-Blodgett Trough (LBT) to study the formation of monolayer and the corresponding phase transitions of these aromatic compounds. The generated comparative results based on ST, LBT, and SHG are currently being analyzed to better characterize the aromatic molecules at the air-water interface.
5. Summary and Impact on Students and the PI
The PRF support facilitated a unique opportunity for us to establish an experimental setup to probe photochemistry at the air-water interface. While the photochemistry of pNP– at the air-aqueous interface is still under investigation, we have also characterized the corresponding bulk photochemistry and identified new molecules to be studied using the photochemistry setup. During this period, one graduate student (Mr. Daniel Headley, MS candidate) has been trained. He is currently continuing with this project supported by the PRF grant. Contribution from his work has been highlighted at a poster session at the 2015 Indiana Academy of Sciences Meeting. In addition to providing financial support for research and supplies, the funding has also supported five undergraduate students (ranging from sophomores to seniors), two during the academic year and three as part of the departmental Chemistry Research Immersion Summer Program. The paid opportunity made it possible for them to learn, experience, and be inspired by the cutting-edge tools and advanced fundamental science.
The PRF funded project has also initiated discussions within the department and among scientists in the nonlinear spectroscopy community for potential collaborative work. Furthermore, based on the preliminary data resulted from this work, I have been able to participate in submitting an interdisciplinary NSF MRI grant), which has been recently funded (Award No. 1531851) for the acquisition of a LCMS unit. Our ongoing effort in elucidating interfacial photochemistry using surface spectroscopy will integrate the powerful trace analysis capability of mass spectrometry.