Reports: ND754633-ND7: Flow-Induced Organization of Bimodal-Sized Particles in Drying Polymer Droplets
Pinar Akcora, Stevens Institute of Technology
Colloidal assembly in polymer solution holds critical importance in creating thin and flexible polymeric devices. Different routes are investigated to control the nanoparticles assembly such as via polymer adsorption or chemical functionalization of particles with polymers. My group works on understanding the aggregation of polymer-functionalized nanoparticles in polymer solutions and bulk films. Polymer adsorption on nanoparticles is currently being studied as new research directions in my group. With the support of this ACS PRF grant this past year, we investigated how polymer adsorbed colloidal particles aggregate during evaporation. We have measured the contact line movement of a polymer droplet to understand and control the ordered deposition of microspheres in the vicinity of polymer chains by controlling the evaporation rate.
Project Results: Droplets of poly(vinylpyrrolidone) (PVP) solutions containing PS micron size beads were examined on a hydrophilic substrate during solvent evaporation with an optical microscope and goniometer. We measured the droplet size and contact angle under slow and free (fast) evaporation. Using the Nikon OPTIPHOT 2POL optical microscope, evaporation-controlled patterns of particles were observed at alternating evaporation rates.
In
a freely evaporating polymer droplet, we have previously shown that particles
and polymer were first carried by radial capillary flow and then spatially
arranged particle stripes were formed due to spontaneous demixing of
polymer-rich and particle-rich phases. Marangoni flows induce the circulatory
motion of particles allowing the growth of stripes in a radial direction. We
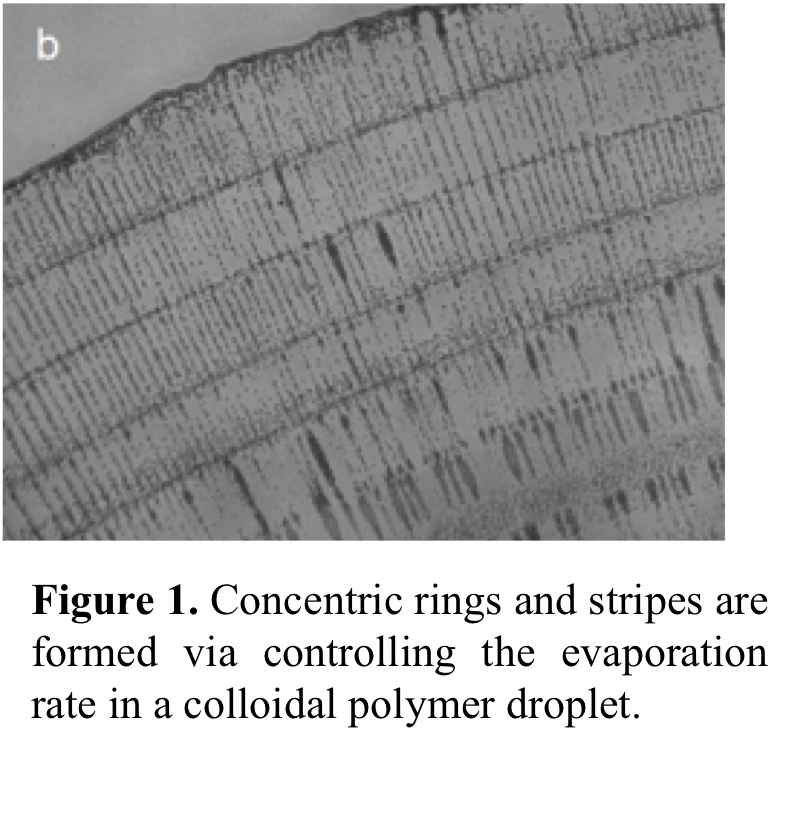
have now found that when the evaporation rate is slowed down, particles start
forming concentric rings as shown in Figure
1. We showed that deposition of particles into concentric rings and stripes
was controlled precisely by varying the evaporation rate. To understand the
formation of these rings, we have analyzed the kinetics of contact line
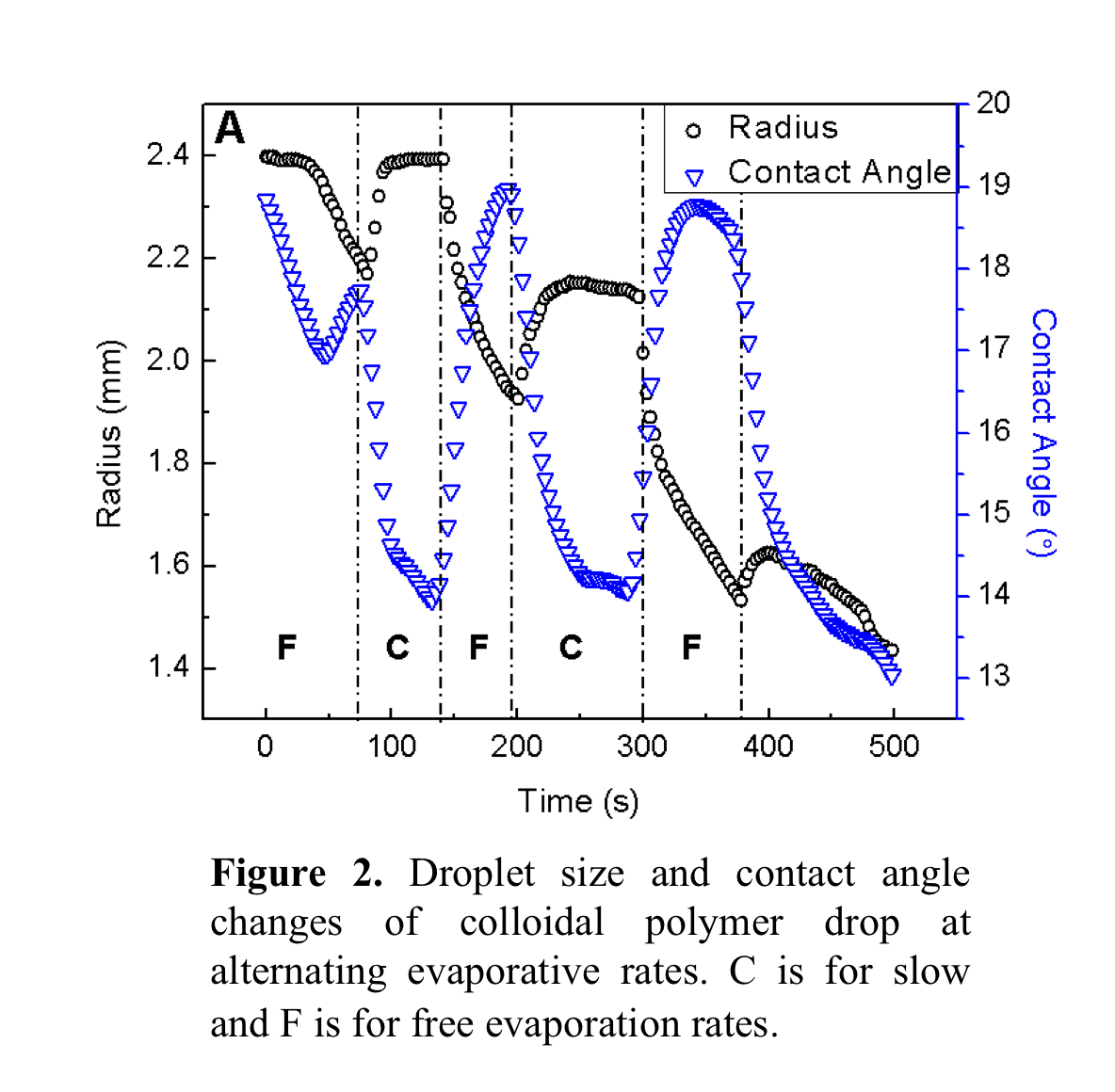
movement. Figure 2 shows that when
evaporation is slow (labeled regions as C), contact line (i.e. droplet size) advances and contact angle decreases. Under free
evaporation, contact line recedes and contact angle increases, which is shown in
the regions labeled as F. It is known that hydrophilic polymer does not pin on
a hydrophilic surface and leaves a thin polymer film that smooths the
heterogeneities of a surface. This thin layer film deposition enables the
contact line to move freely during evaporation leaving particles in striped
patterns. The spreading of droplet is defined by the fluid velocity parallel to
the surface, UCL, and can
be determined as a function of contact angle (q), given by UCL=nf(q). Mass balance of a droplet is conserved
with the spreading of solution and evaporation rate, J(r). Thus, velocity of a contact line, dr/dt, is defined as dr/dt=UCL-J(r)/[rLsinq(t)] {Equation (1)}.
Assuming that evaporation rate J(r) is
uniform, J can be calculated from the
volume change of droplet by J=dV/dt.
The droplet volume V is calculated
using the following equation
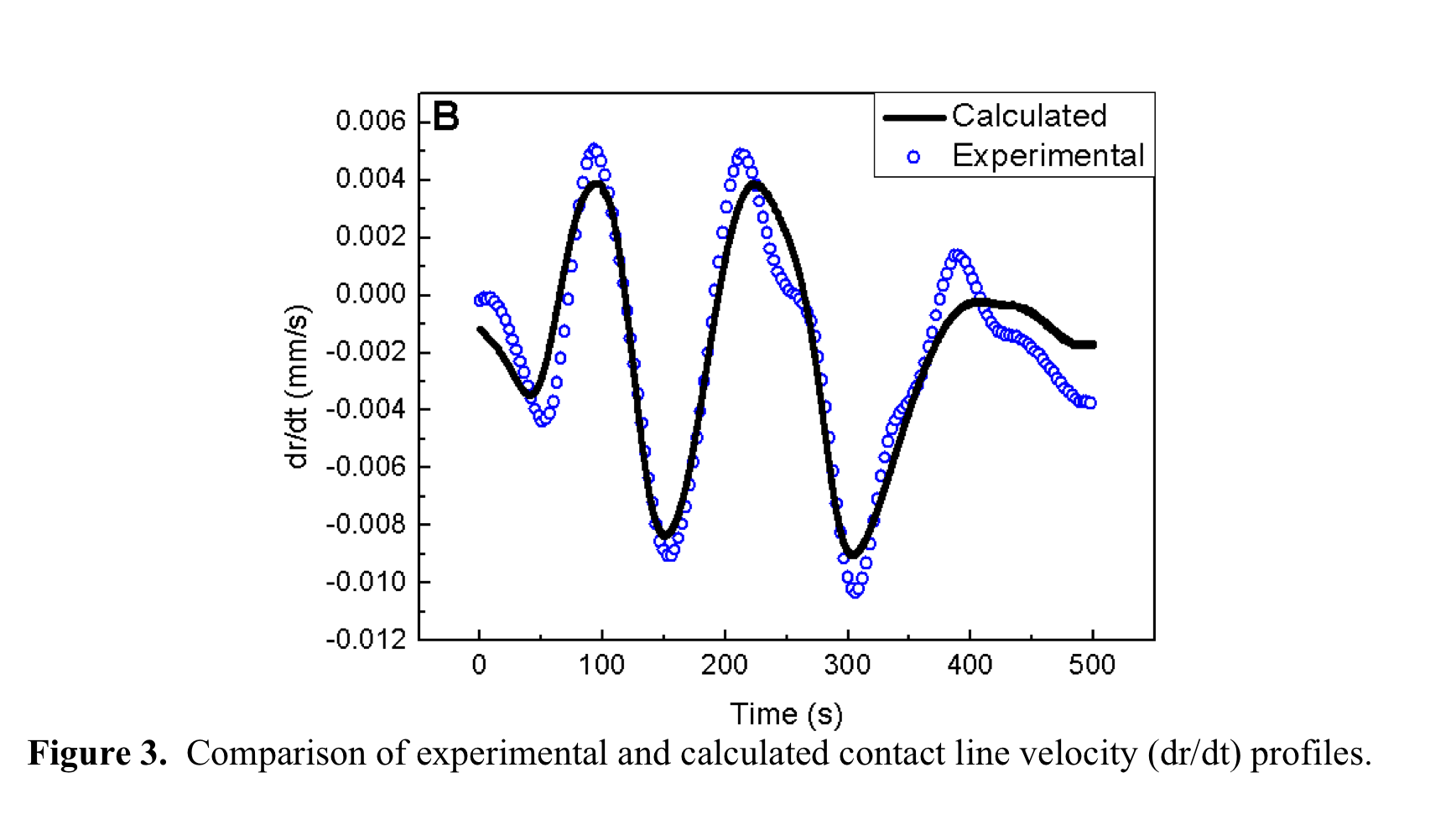
![]() . Our experimental contact
line dynamic data is presented in Figure
3. Using the experimental data r
and q as functions of evaporation
time, we have calculated J and
determined the value of UCL
to fit to the experimental data of dr/dt. We found that flow velocity parallel to the
substrate is constant (UCL=0.00433 mm/s) except the
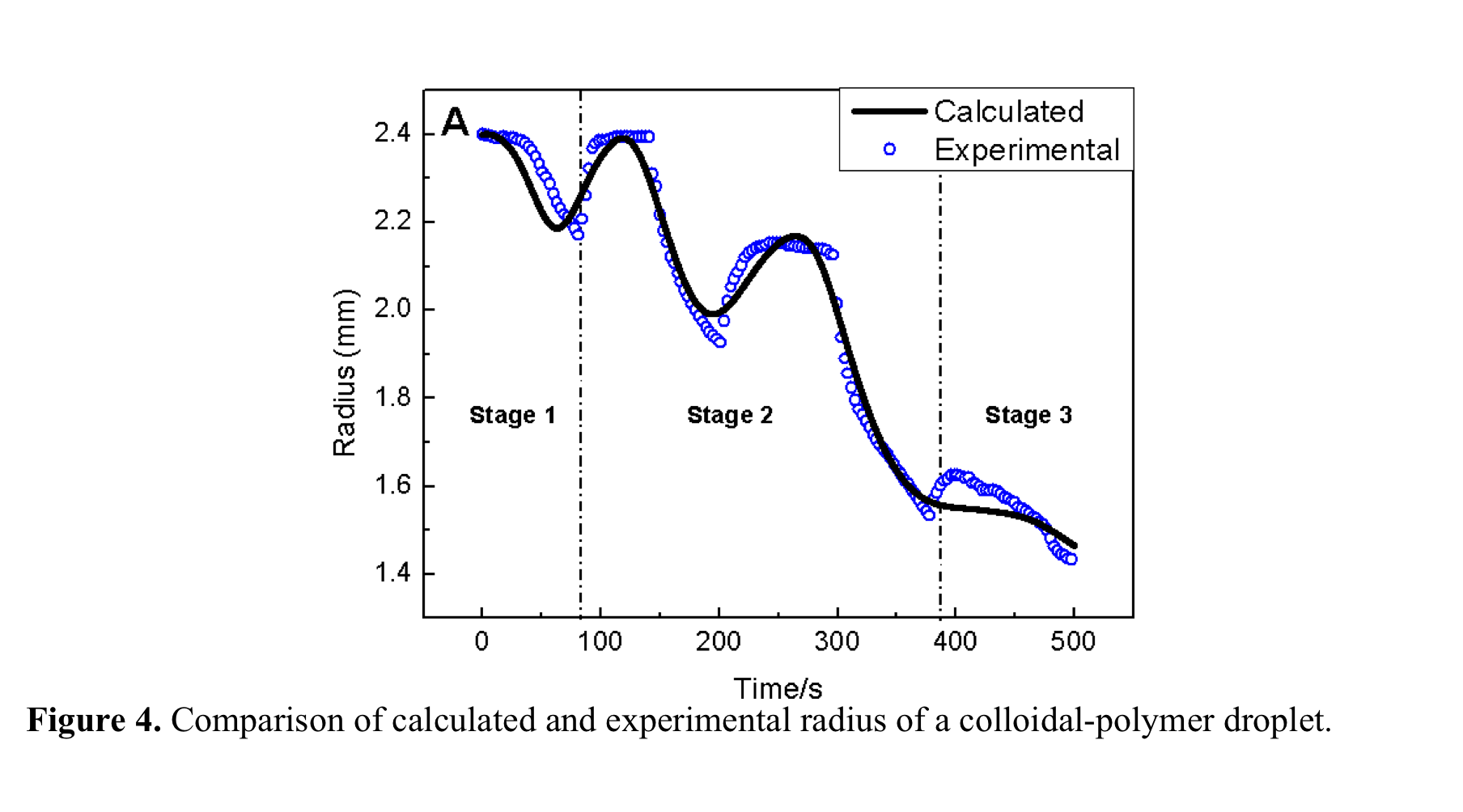
initial pinning and final evaporation stages. Stage 2 in Figure 4 shows the fitting between the model (Equation 1) and data
of radius of a droplet where ring formation and stripes are formed in alternating
evaporation rates. We have shown in our earlier work that in free evaporation,
capillary flow is balanced with Marangoni flow where continuous growth of
stripes occurs and during this time UCL
remains constant. Consequently, droplet size is varied just by controlling the
evaporation rate. When UCL
is equal to evaporation rate J, that
is when dr/dt=0 and contact line is stationary, particles which continuously are
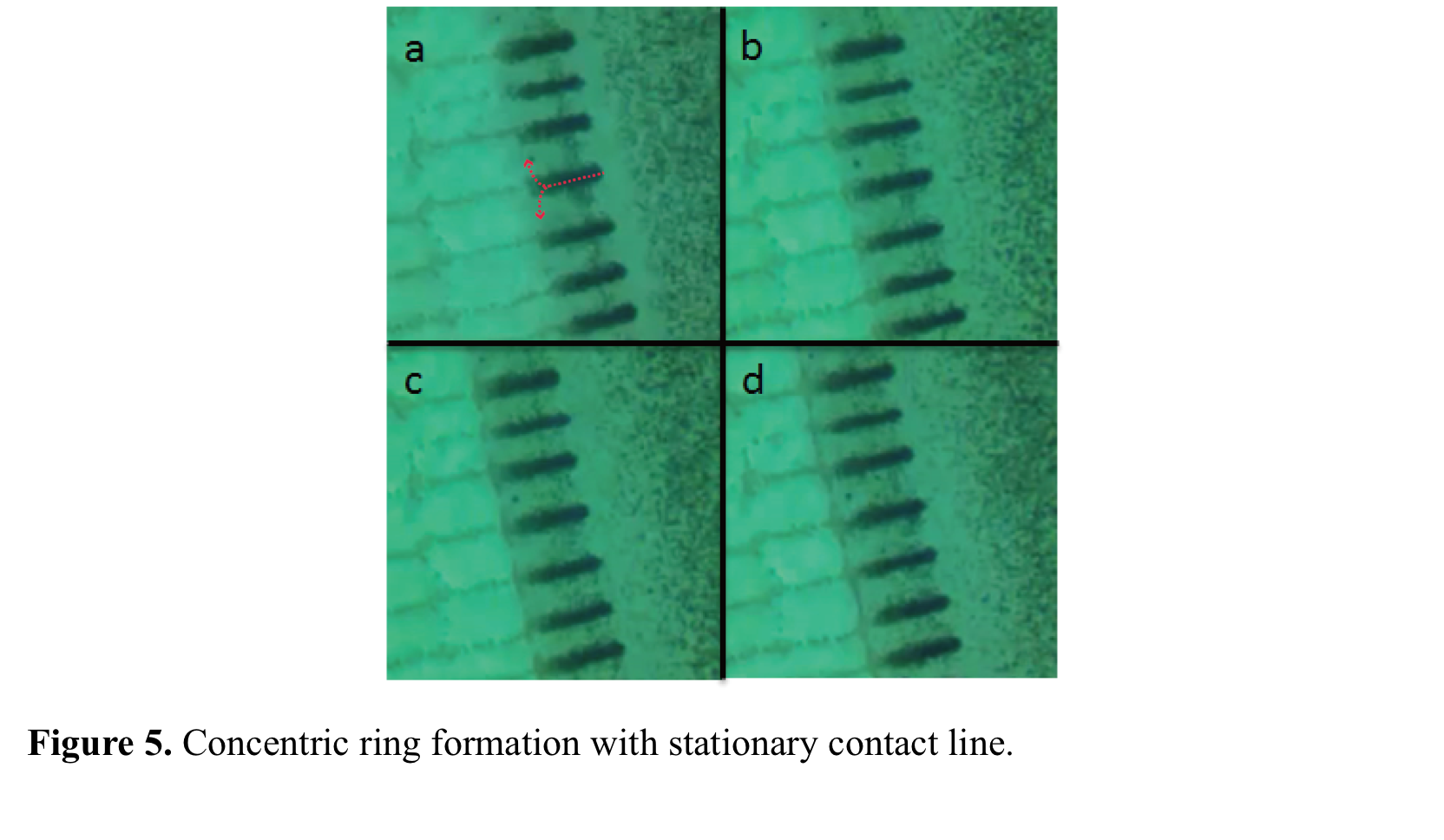
replenished by the stripes, start to accumulate as a ring. Figure 5 shows the snapshots of a ring with stationary contact
line. Concentric deposition
of particles occurs with the balance between evaporation rate, J, and UCL but not due to the pinning-depinning of the contact
line. The width of each concentric ring is adjusted by stationary time and the
distance between concentric rings is tuned with receding time. The manuscript
summarizing the dynamics of assembly of microspherical particles is in preparation
for submission to Applied Physics Letters. Additionally, we worked on the
bimodal sized particles of microspheres mixed with 100 nm PS spheres at 10:1
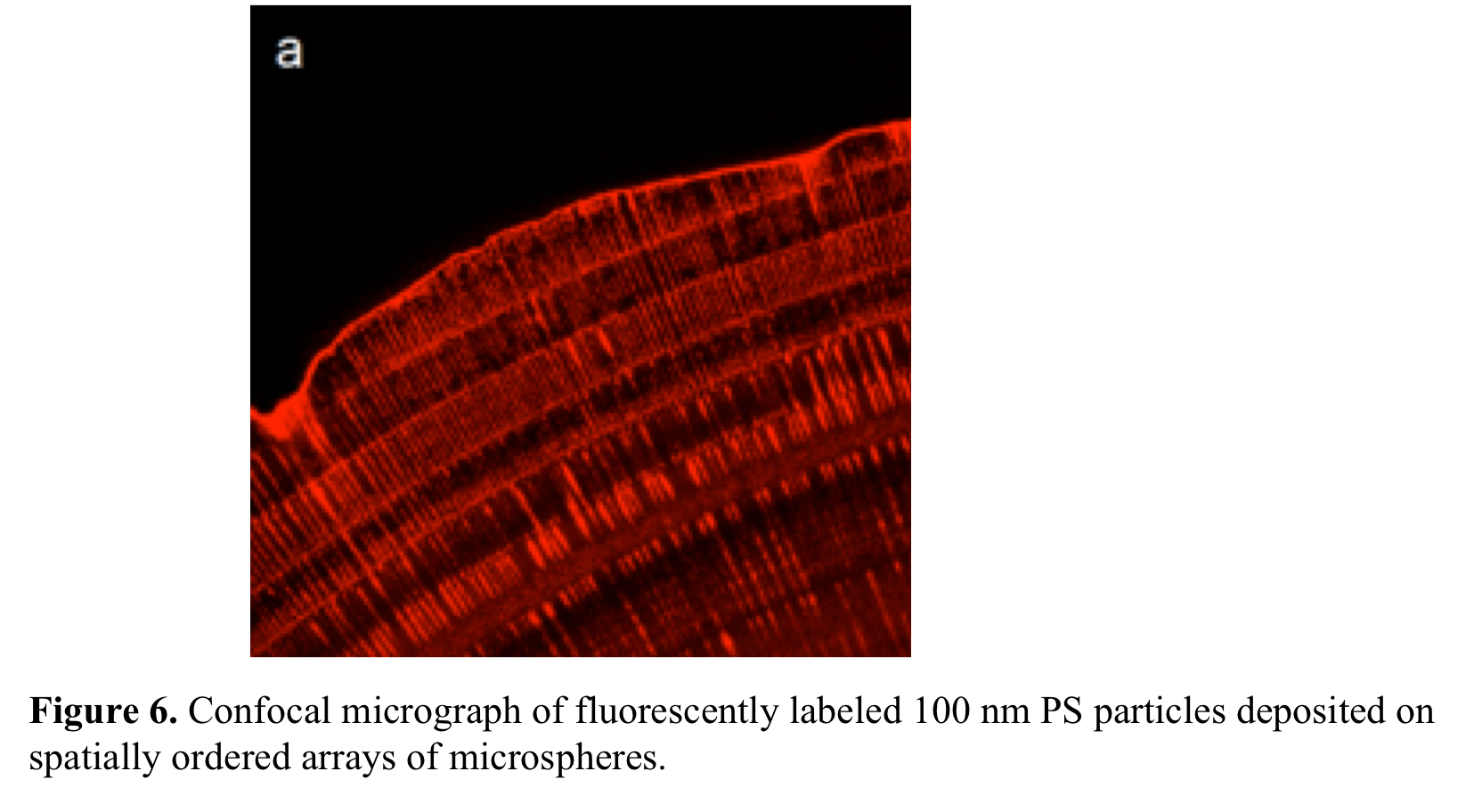
and 10:2 vol:vol ratios. Figure 6
shows that fluorescently labeled nanoparticles deposit onto obtained patterns
of microspheres.
. Our experimental contact
line dynamic data is presented in Figure
3. Using the experimental data r
and q as functions of evaporation
time, we have calculated J and
determined the value of UCL
to fit to the experimental data of dr/dt. We found that flow velocity parallel to the
substrate is constant (UCL=0.00433 mm/s) except the
initial pinning and final evaporation stages. Stage 2 in Figure 4 shows the fitting between the model (Equation 1) and data
of radius of a droplet where ring formation and stripes are formed in alternating
evaporation rates. We have shown in our earlier work that in free evaporation,
capillary flow is balanced with Marangoni flow where continuous growth of
stripes occurs and during this time UCL
remains constant. Consequently, droplet size is varied just by controlling the
evaporation rate. When UCL
is equal to evaporation rate J, that
is when dr/dt=0 and contact line is stationary, particles which continuously are
replenished by the stripes, start to accumulate as a ring. Figure 5 shows the snapshots of a ring with stationary contact
line. Concentric deposition
of particles occurs with the balance between evaporation rate, J, and UCL but not due to the pinning-depinning of the contact
line. The width of each concentric ring is adjusted by stationary time and the
distance between concentric rings is tuned with receding time. The manuscript
summarizing the dynamics of assembly of microspherical particles is in preparation
for submission to Applied Physics Letters. Additionally, we worked on the
bimodal sized particles of microspheres mixed with 100 nm PS spheres at 10:1
and 10:2 vol:vol ratios. Figure 6
shows that fluorescently labeled nanoparticles deposit onto obtained patterns
of microspheres.
In summary, the evaporation controlled ordering of bimodal sized particles into ordered patterns is governed by the dynamics of microspheres which serve as dynamic templates for the assembly of periodic arrays of nanoparticles. The reported method can be an alternative technology to ink-jet printing to control deposition of colloids in polymer films or substrates.
We are currently working on the PS beads (100 nm), anisotropic particles, and smaller (50 nm) SiO2 nanoparticles mixtures in PVP to obtain nanoparticle arrays on surfaces after washing off the PS latex particles. Experiments on PAA grafted nanoparticles are also underway.
Impact on Students: This funding has helped us to design experiments to understand substrate-particle-polymer interactions and evaporation kinetics on preparing functional film devices for thin film applications and beyond. A new Ph.D. student (Chongfeng Zhang) with a B.S. degree in Polymer Science and Engineering is being supported by this grant and his training on colloidal phenomena and polymer science helps greatly to this project. Another Ph.D. student in my group (Erkan Senses, graduated in Spring-2015) has worked on the aggregation of polymer adsorbed iron oxide nanoparticles under evaporation.