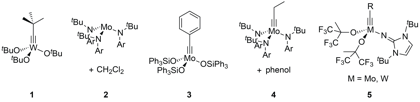
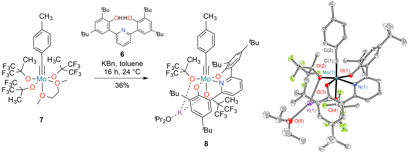
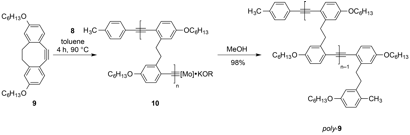
Reports: DNI752722-DNI7: Ruthenium and Osmium Alkylidyne Complexes as Catalysts/Initiators for the Ring-Opening Alkyne Metathesis Polymerization
Felix R. Fischer, PhD, University of California Berkeley
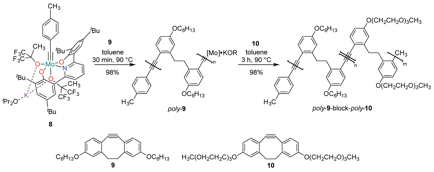
We studied the ROAMP of 3,8-dihexyloxy-5,6-dihydro-11,12-didehydrodibenzo[
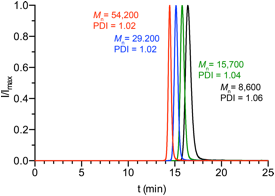
[ T (°C) theory GPC GPC PDI GPC 10 90 4,000 8,600 9,200 11 1.06 20 90 8,100 15,700 16,200 23 1.04 50 90 20,200 29,200 30,000 47 1.02 100 90 40,400 54,200 55,300 99 1.02
[ T (°C) theory GPC GPC PDI GPC 0/10/1 90 5,400 5,700 6,100 0/9 1.08 10/0/1 90 4,000 3,300 3,800 10/0 1.15 10/10/1 90 8,300 11,000 11,800 11/12 1.07 20/0/1 90 8,100 14,400 15,000 20/0 1.04 20/20/1 90 13,400 25,400 27,200 20/20 1.07