Reports: ND554519-ND5: Synthesis and Surface Reactions of Organometallic Precursors Tailored for Electron Beam Induced Deposition
Lisa McElwee-White, PhD, University of Florida
D. Howard Fairbrother, PhD, Johns Hopkins University
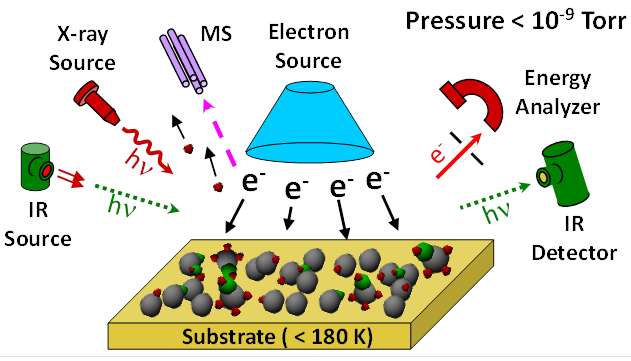
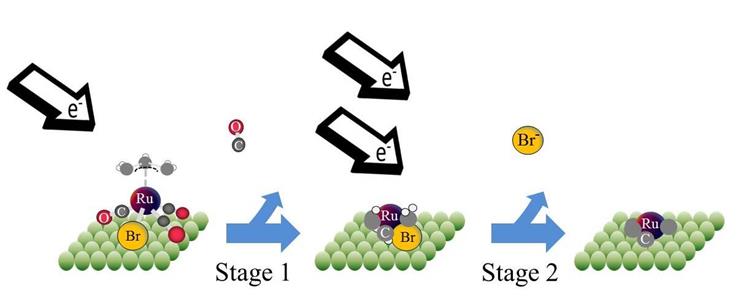
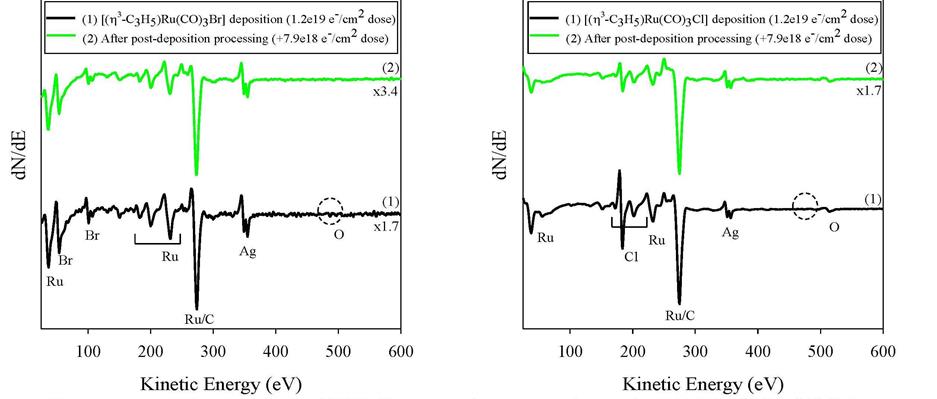
Lisa McElwee-White, PhD, University of Florida
D. Howard Fairbrother, PhD, Johns Hopkins University



Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society