Reports: DNI1054775-DNI10: Design of Green, Switchable Oxide Catalysts for Steam Methane Reforming by Quantum Mechanical Simulation
Elif Ertekin, University of Illinois
Executive Summary
Steam methane reforming is the most widely-used industrial process for hydrogen and synthesis gas production from fossil fuels, and currently accounts for over 95% of the hydrogen produced in the United States. As part of our ACS-PRF program, we are exploring and establishing a new approach to tailored design of catalysts for steam methane reforming based on the use of metal catalysts integrated with ferroelectric oxide support layers, in which dynamic switching of the polarization of the support layer is used in real time to modify surface properties, and hence promote the desired chemical reactions, on the metal catalyst surface (see FIG. 1). The approach is based on polarization modulation of a ferroelectric oxide support to reduce key reaction barriers for molecular adsorption, dissociation, association, and release, and hence promote desired pathways. Using first-principles quantum mechanical modeling methods based on density functional theory, the goals of this program are to (i) identify the mechanisms and rate limiting steps for steam methane reforming reactions on nickel catalysts, (ii) design integrated ferroelectric oxide/metal systems in which the polarization tunes the electronic structure and surface chemistry of the metal, and (iii) optimize the design and assess the ultimate potential of the newly proposed mechanism. Alongside the research, the program will train undergraduate and graduate students in the atomistic modeling and simulation of chemical processes relevant to the petroleum field.
Introduction
Steam methane reforming is currently the most widely used process for hydrogen production in the United States. It is a highly endothermic reaction (requiring an external energy supply) that uses high-temperature steam to convert two stable molecules (methane to hydrogen) to the more reactive carbon monoxide gas:
CH4 + H2O → CO + 3H2(DH = -206 kJ/mol). (1)
Widescale adoption of this process is currently hindered by challenges in capital costs and operation and maintenance costs of the steam methane reforming process. As their costs preclude the usage of highly-active, stable noble metal catalysts on the industrial scale, nickel is the most widely-used catalyst today. However, conversion efficiencies of nickel catalysts are not as high as their noble metal counterparts, and their performance is severely hindered by coking, ultimately leading to encapsulation and deactivation of the catalyst. There are unexplored opportunities in the design of oxide-supported metal catalysts for steam methane reforming. Amongst the class of oxides considered as catalytic support layers, ferroelectric oxides are currently receiving attention on account of their switchable polarization, which may offer routes to dynamic control over surface chemistry and chemical reactions. If successful, the development of high-efficiency catalysts based on this approach will provide a strong incentive to develop on-site infrastructures to capture and convert natural gas into products useful for society.
Methods & Results
In the first year of this program, we have carried out first principles electronic structure calculations to trace the steam methane reforming reaction taking place on exposed nickel surfaces that are supported by ferroelectric oxide substrates. Some of the most exciting highlights of our work from the previous year are:
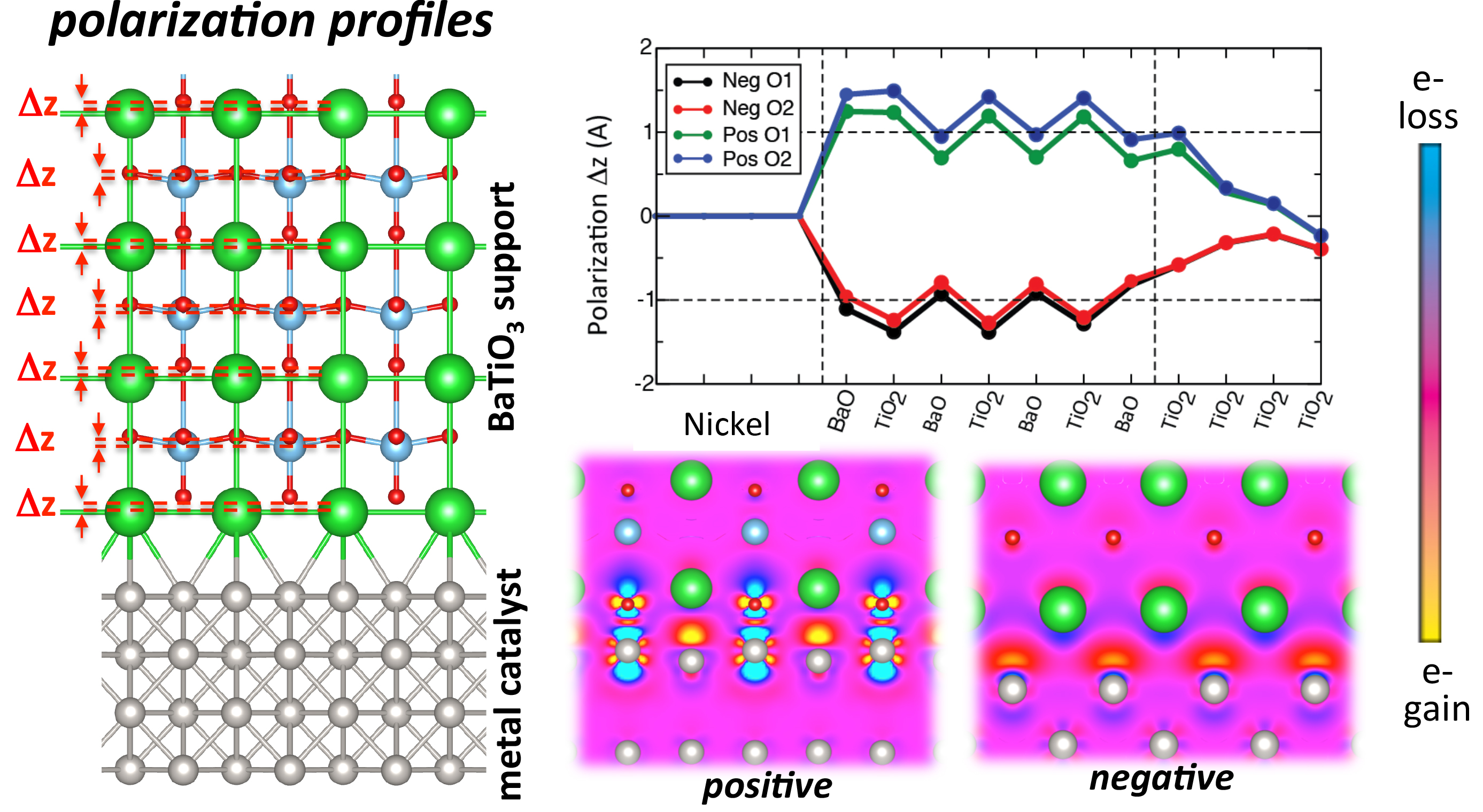
- Simulation of nickel surfaces interfaced with barium titanate ferroelectric layers for both positive and negatively polarized support layer. Our simulations have revealed the energetically preferred orientation of layers of nickel catalyst interfaced with the ferroelectric material BaTiO3, for the case of positively and negatively polarized BaTiO3. This orientation is shown in Fig. 1 (for the case of positive polarization).
- Assessment of the resultant polarization profile of the ferroelectric. The resultant polarization of the ferroelectric material has been determined for both polarizations, and demonstrated to recover degrees of polarization comparable to the bulk ferroelectric material for oxide thicknesses that are several unit cells thick. These profiles are plotted in Figure 1.
- Analysis of interface dipole for both polarizations. The degree of interfacial charge rearrangement due to interface formation (for both polarizations) and the resulting interface dipole, have been established. These are illustrated in the charge density maps for both polarizations of ferroelectric material. The different degrees of charge rearrangement result in different degrees of charge transfer, demonstrating that the electron richness of the catalyst layer can be tuned by this polarization.
Plans for Year Two
In our first year, we focused on creating accurate representations of the integrated ferroelectric oxide/catalyst system, which will serve as the template for the analysis of the chemical reaction of interest. In the second year of the program, we will carry out the analysis of the full steam methane reforming reaction using these integrated ferroelectric/Ni systems, for the cases of both positively and negatively polarized ferroelectrics. The chemical reaction pathways and barriers will be compared to those obtained for isolated nickel catalyst layers. We expect that the oxidation and reduction reactions involved in the full cycle will be affected by the surface electron availability, suggesting that dynamically switching the polarization may be a useful approach for overcoming key reaction barriers and facilitating the forward reaction.
Fig. 1. Total energy electronic structure methods based on density functional theory demonstrate that ultra thin Nickel catalyst layers supported by a polarizable oxide substrate (BaTiO3) offer routes for tuning chemical reaction pathways and landscapes. Switching the polarization direction of the oxide between positive and negative results in the formation of distinct electrostatic dipoles at the oxide/metal interface (charge density maps). The resulting change in the surface chemistry can result in modifications to the energy landscape of the steam-methane reforming reaction.