Reports: DNI1054190-DNI10: A Novel Integrated Design for the Mixed Ionic-Electronic Conducting (MIEC) Perovskites
Yu Zhong, Florida International University
State of the Art: Electronic and oxygen-ionic conductivities are directly linked with the performance of Oxygen Transport Membrane (OTM). There are well-established theories on the relationship between defect chemistry and electronic and ionic conductivities. For example, the electronic conductivity can be expressed as:
σe = σn + σp = enμn + epμp (1)
Similarly, the oxygen-ionic conductivity is related to the concentration of the carrier, i.e. oxygen vacancy ( VÖ), through the following equation:
σO = 2e[VÖ]μVÖ (2)
Problem Statement: Over the past two decades, extensive effort has been carried out on the electrical conductivities modeling for perovskites. The two sets of critical parameters are the carrier concentration, i.e. n, p, and [VÖ], and the mobilities, i.e. μn, μp, and μVÖ . Traditionally, the carrier concentrations were achieved from various semi-empirical defect models, which were developed based on a simplified relationship of the concentrations of elements or ions. The mobilities would then be achieved with the curve fitting by using experimental measured conductivities and the carrier concentrations. Its main problem is it cannot be applied to the experimental unexplored compositions, temperatures, and p(O2) because:
1.) The simplified relationship of the concentrations of elements or ions may cause significant error in mobilities, even though a good match with the measured conductivity can be achieved with curve fitting.
2.) The carrier concentration is unknown in the experimental unexplored regions. The carrier concentration can be very different with the change of compositions, temperatures, and p(O2).
Without the reliable carrier concentration, the traditional approach lacks the capability to predict electrical conductivities, especially for the experimentally unexplored or unachievable regions. This obstacle greatly hindered the discovery of valuable novel metal oxides.
On the other hand, several efforts in the CALPHAD community, including the one from the PI, have been made to develop the thermodynamic databases for metal oxides using the computational thermodynamics approach, especially for perovskites. It covers the defect chemistry for the whole composition range of perovskites. More important, it is able to predict the quantitative Brouwer diagrams, which were proposed by the PI.
Proposed Tasks: The quantitative Brouwer diagram will be developed with the LSCrF thermodynamic database. It will be used to provide the carrier concentrations, i.e. n, p, and [VÖ]. They will be used along with the experimental electrical conductivities from the transport measurement to calculate the mobilities, i.e. μn, μp, and μVÖ. The investigation will start with the most popular LSM and LSCrF perovskites. It will be expanded to provide a predictive map of the electronic and oxygen ionic conductivities of the complete composition range for LSCF perovskites.
Task 1 Quantitative Brouwer Diagram Predictions. In the current proposal, by using the thermodynamic database, the quantitative defect chemistry analysis can be carried out for the selected LSCrF perovskites. The quantitative relationship between the concentration of the ionic species (including the concentration of carriers n, p, and [VÖ]) and various parameters, such as stoichiometry, p(O2), and temperature, will be established.
Task 2 Conductivity Measurements. As the existing experimental conductivity data are limited, our transport measurements will be carefully designed to collect the key experimental data.
Task 3 Conductivity Predictions. With the carrier concentration from Task 1 and the conductivity measured from Task 2, the mobilities at different conditions will be determined. The behavior of the ionic and electronic conductivities will be plotted as a function of dopant concentration, annealing temperature, and p(O2) during high-temperature annealing. Through incorporation with the quantitative defect chemistry information from the La-Sr-Cr-Fe-O thermodynamic database, a predictive map of the electronic and ionic conductivities of the complete composition range for LSCrF perovskites will be established.
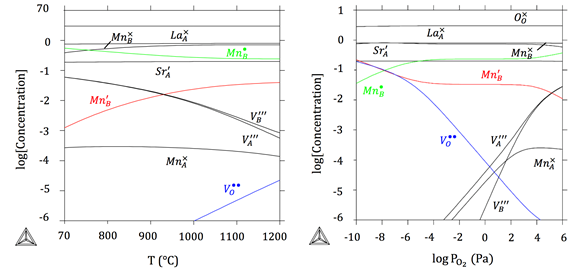
Prior Work: The PI has developed the quantitative Brouwer diagrams based on the CALPHAD approach of a similar perovskite LSM showing the relationship between the species’ concentration and temperature (left) and p(O2) (right), as shown in Figure 1, with the same CALPHAD approach proposed above. LSM was modeled as (La3+,Mn3+,Sr2+,Va)1(Mn2+,Mn3+,Mn4+,Va)1(O2-,Va)3, where La3+,Mn3+,Sr2+ and vacancy occupy the A site, Mn2+,Mn3+,Mn4+ and vacancy occupy the B site, and O2- and vacancy occupy the O site. Specifically, it clearly shows the concentration of [Mn′B] in red (the carrier n), [Mn⋅B] in green (the carrier p), and [VÖ] in blue, which were labeled in color. It can be easily seen that p>>n in most cases. That is why LSM is typically treated as the p-type conductor.
Figure 1. The quantitative Brouwer diagrams of LSM20. The concentration of species at different temperatures in air (left) and at different p(O2) at 1000oC(right).
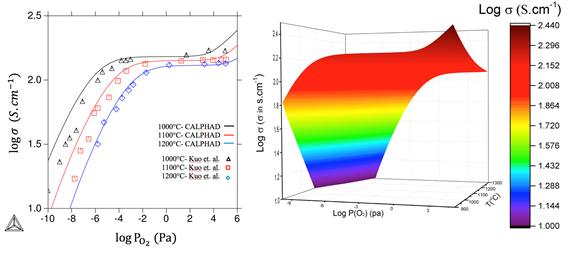
With this approach, recent success has been achieved by the PI in the electronic conductivity prediction for the p-type LSM, as shown in Figure 2. Electron hole mobility μp can be achieved by the electronic conductivity measured at different temperatures. Based on the quantitative Brouwer diagram for the carrier p ([Mn⋅B]) at 1273 K, 1373 K, and 1473 K in a wide p(O2) range, the electronic conductivity was recently predicted by the PI. Note that Figure 2 shows excellent agreement with the experimental observations from Kuo et al. (left).
Figure 2. Experimental and calculated electronic conductivity (σ) of La0.8Sr0.2MnO3±δ, vs. PO2 (left); 3-D contour of electronic conductivity as a function of p(O2) and temperature (right).
The 3-D contour of electronic conductivity as a function of oxygen partial pressure and temperature was also predicted (right). In the case of ionic conductivity, the ionic conductivity of LSM20 was determined to be as low as 5.9x10-8S/cm, which can be explained by our quantitative prediction of extremely low [VÖ] (Figure 1). That is the reason why LSM is considered as a pure electronic conductor.