Reports: ND553999-ND5: Electron-Phonon Interactions in Atomically Monodispersed Gold Cluster/Graphene Oxide Nanocatalysts
Ramakrishna Guda, Western Michigan University
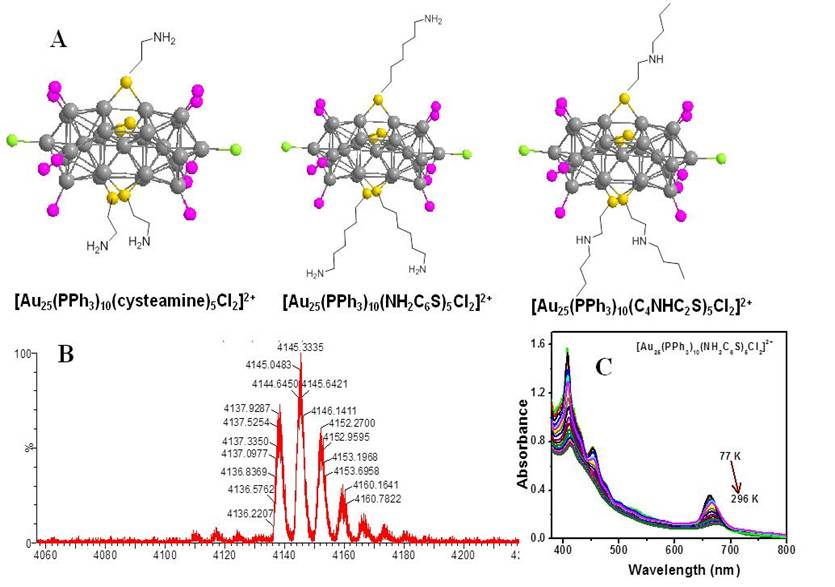
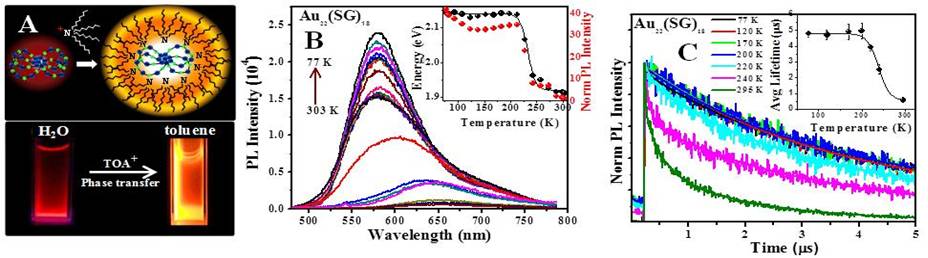
Ramakrishna Guda, Western Michigan University


Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society