Reports: UNI352868-UNI3: Alternative Clean Energy Production with Reusable Iron Catalysts: Development of Design Parameters for Future, Durable Green Energy Catalysts
Jesse W. Tye, PhD, Ball State University
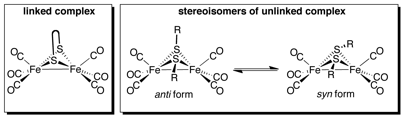
Jesse W. Tye, PhD, Ball State University

Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society