Reports: UR152162-UR1: Development and Application of Air-Stable, Shvo-Type Iron Catalysts
Timothy W. Funk, PhD, Gettysburg College
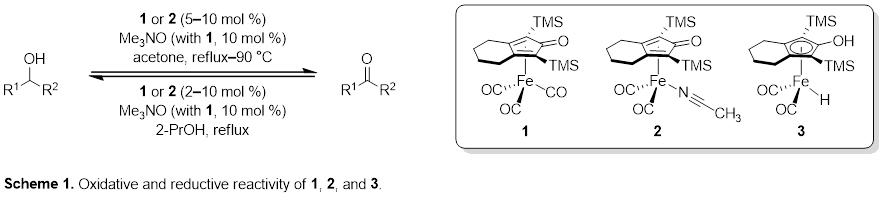
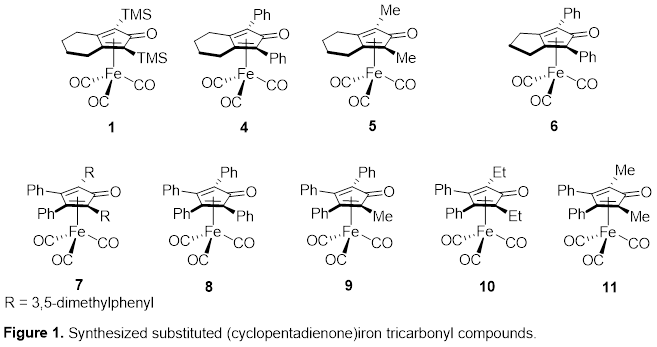
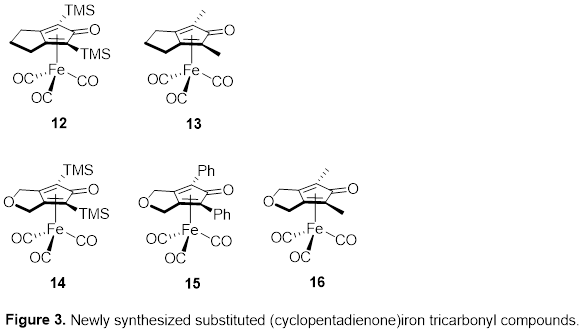

Timothy W. Funk, PhD, Gettysburg College




Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society