Reports: UR453159-UR4: Impact of Solvent-Solute Interactions on Photochemistry of p-Aminobenzoic Acid Derivatives
Sarah J. Schmidtke Sobeck, PhD, College of Wooster
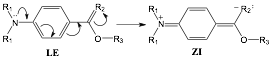
We are interested in understanding how perturbations in the environment and nature of the donor-acceptor moieties impact the photochemistry of potential charge transfer systems. A general scheme for the intramolecular charge transfer (ICT) that may occur following photo-excitation in our model system, PABA, is shown in Figure 1. During the first year of ACS-PRF funding we focused upon comparing the photophysical properties of p-aminobenzoic acid (PABA, R1=R3=H, R2=O) and dimethyl-p-aminobenzoic acid (DABA, R1=CH3, R3=H, R2=O) in a range of solvents. The results were prepared and published during the current year. In the present grant year, our work has focused upon continuing to quantify physicochemical properties of PABA derivatives, synthesis of molecules with variations at the electron acceptor moiety of the ICT system, and initiating photodegradation studies of the parent acids.
Figure 1. Potential ICT reaction from the locally excited (LE) to zwitterionic (ZI) states for PABA derivatives.
Results
This past academic year, 2014-15, the PI was on an academic leave and spent five months at Yale University working in the lab of Dr. Paul Whitmore at the Institute for the Preservation of Cultural Heritage. At Yale a project was initialized analyzing the impact of the paint media and ambient environment on the photo-degradation of cochineal (carmine) pigment. A significant amount the time was used to learn techniques for preparation and analysis of solid-state samples. A portion of the leave was also dedicated to finalizing experimental work and writing up the results of the work described in our 2013-14 report, and resulted in an article that included the work of three students in our lab. The quantum efficiencies for PABA and DABA in a range of solvents, and the thermodynamics for the ICT reaction of DABA in the solvents were summarized in the paper. The results of the PABA studies were also included in a presentation at the IUPAC World Chemistry Congress in Busan, Korea during August.
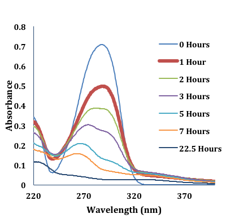
During the summer of 2015, the PI returned to her home institution and advised three research students on two projects related to the PABA studies. One project, led by student researcher Preetom Borah, focused upon the UV-induced degradation of PABA and padimate-O, an ester derivative of DABA (R1=CH3, R2=O, R3=C8H17). Both molecules are FDA-approved UV-absorbers for use in personal care products. Padimate-O exhibits ICT following photo-excitation, whereas PABA does not. Previously, it was observed that alcohol-based PABA solutions would discolor with significant light exposure. For this reason, we proposed to follow the photodegradation of PABA and Padimate-O in different solvent environments. Acetonitrile and methanol were initially selected as solvents to compare protic and aprotic environments. Solutions are irradiated in a UV-light chamber and the absorbance monitored throughout the process, Figure 2, to allow kinetic analysis of the degradation. Initial LC-MS analysis of the irradiated solutions shows new peaks, assigned to photoproducts, that vary in number and mass depending upon the solvent.
Figure 2. Representative change in the absorbance of PABA in methanol as a function of UVA-irradiation time.
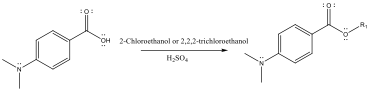
A second project focused on the synthesis of PABA derivatives with variations at the electron acceptor moiety. Haley Rossiter, a rising in junior, led this work. Haley synthesized several ester derivatives of DABA, to explore the impact of the ICT acceptor moiety on photophysical properties. Five derivatives were prepared, including esters (R3=C2H5), chloroesters (R3=C2H3Cl, C2H2Cl3), and thionesters (R2=S), using procedures detailed in Figures 3-4. The purification, optimization of reaction conditions, and characterization of the five compounds is ongoing. We did find that esterification slightly reduces the degree of ICT, and chlorination of the ester group increases the degree of ICT. Unfortunately, the thionoesters are not fluorescent so the ICT process cannot be traced, but thionation of the ester leads to a significant red shifts in the absorbance.
Figure 3: Sample esterification reaction for synthesis of esters and chloroesters of DABA.
Figure 4: Thionation reaction to yield thionester derivatives of the ester and chloroesters of DABA.
A third summer research student, Erin Drake, built a larger scale UV-exposure chamber with UVA and UVB light banks. She tested methods to analyze solid samples that are not transparent and to follow chemical changes of such samples throughout an irradiation experiment. Preliminary results indicate that reflectance measurements, using the solid-state fluorimeter accessory, are a promising quantitative method. This work may lead to future applications of studying photochemistry of UV-absorber coated surfaces.
Additionally, the PI traveled to The Ohio State University to take preliminary time-resolved fluorescence measurements in the lab of Prof. Claudia Turro. Some of the laser equipment that we intended to use is currently not working, so tests were carried out on another instrument. The measurements show promise for capturing fluorescence lifetimes of some of the PABA compounds and future trips with students are intended in the coming year.
Ongoing Plans
The summer research students plan to submit abstracts to present posters at the National American Chemical Society meeting in spring, and will present the work at campus-wide events. During the present academic year Haley Rossiter is continuing the synthesis of PABA derivatives, optimizing synthesis conditions and characterizing the products. The results of this work are being assembled into a manuscript. Additionally, the PI is advising four Senior Thesis students. Two of the students, including Preetom Borah, will be analyzing the photodegradation of PABA in different solvents and pH environments. The other two students will be analyzing the lightfastness of carminic acid in solution and carmine paints.
The PI and her group are very appreciative of the support of this work from the ACS PRF. This support is vital in allowing our undergraduates the opportunity to engage in the complete research process.