Reports: UR453166-UR4: Solid-State Chemistry of the Nitrile Oxides
William Hubert Ojala, University of St. Thomas
Nitrile oxides are useful in 1,3-dipolar cycloaddition reactions, yielding a variety of heterocyclic products. In solution, and depending on the reaction conditions, they also form dimers: the furoxan, the dioxadiazine, and the oxadiazole N-oxide (Figure 1). In the second summer research season of our ACS-PRF grant, we continued to examine crystalline nitrile oxides to determine: (1) Does nitrile oxide dimerization occur in the solid state? (2) Which of the three dimers is(are) formed as the result of solid-state dimerization? (3) Do different polymorphs of the same nitrile oxide yield different solid-state dimerization products? (4) Given that hindered nitrile oxides do not dimerize in solution but instead rearrange to isocyanates, do hindered nitrile oxides also dimerize to isocyanates in the solid state? (5) Can nitrile oxides be co-crystallized with 1,3-dipolarophiles and be induced to undergo 1,3-dipolar cycloadditions in the solid state to yield products different from those obtained in solution?
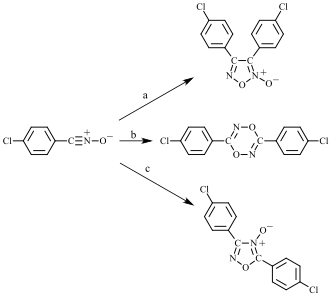
Figure 1. Dimerization of a nitrile oxide (shown here for 4-chlorobenzonitrile oxide) in solution can yield a furoxan (top), dioxadiazine (middle), or oxadiazole-4-oxide (bottom), depending on the reaction conditions: a = inert solvent; b = pyridine-catalyzed; c = trimethylamine-catalyzed
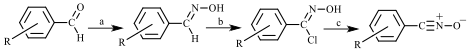
We continued to follow a standard method for synthesizing our nitrile oxides, one that involves chlorination of the substituted benzaldoxime followed by dehydrohalogenation in base (Figure 2). Upon obtaining a nitrile oxide in crystalline form (a task complicated by the tendency of the nitrile oxide to dimerize before crystallizing and by the high solubility of these compounds in the crystallization solvent, usually diethyl ether), we sought to determine its crystal structure by single-crystal X-ray diffraction. We also pursued crystal structure analyses of the various dimers as well as their authentic infrared spectra (and the infrared spectra of the parent nitrile oxides) so that ultimately we can unequivocally determine the identity of any dimer or dimers formed in a solid-state dimerization of a given crystalline nitrile oxide. In the first year of our ACS-PRF grant, Summer 2014, we successfully determined the crystal structures of 4-chlorobenzonitrile oxide and bis(3-bromophenyl)furoxan. Now, as a result of our second-year work in Summer 2015, we have crystal structures for three more nitrile oxides: 3-bromobenzonitrile oxide, 2,3-dichlorobenzonitrile oxide, and 2,6-dichlorobenzonitrile oxide; two more furoxans: bis(4-methylphenyl)furoxan and bis(2,3-dichlorophenyl)furoxan; and three dioxadiazines: bis(3-bromophenyl)dioxadiazine, bis(4-bromophenyl)dioxadiazine, and bis(4-methylphenyl)dioxadiazine.
Figure 2. Typical synthetic route to a benzonitrile oxide from a benzaldehyde derivative;
a = hydroxylamine hydrochloride, base; b = N-chlorosuccinimide; c = aqueous base.
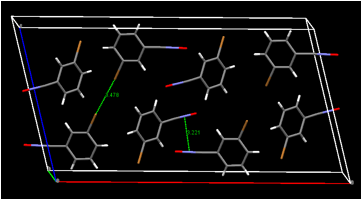
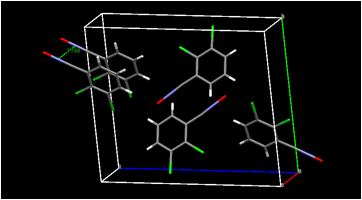
Our crystal structure determination of 4-chlorobenzonitrile oxide in Summer 2014 revealed a close contact between the negatively charged oxygen atom of the fulminate group and the chlorine atom of a neighboring molecule. This raised the possibility that a robust interaction of this type could be important in the crystal engineering of nitrile oxides; moving the halogen atom to different ring positions while leaving the oxygenÉhalogen interaction intact might lead to different packing motifs that would in turn lead to different solid-state dimerization products. Our work in Summer 2015 has now demonstrated that the oxygenÉhalogen contact is not robust. It is absent from our three recent nitrile oxide crystal structures (Figure 3). On the other hand, halogenÉhalogen contacts, also relevant to crystal engineering, are present in two of our three nitrile oxide structures. Our limited number of nitrile oxide crystal structures does not allow us to state definitively whether halogenÉhalogen contacts in nitrile oxide crystals might be generally useful supramolecular synthons for crystal engineering of nitrile oxides, but we intend to examine that possibility in the coming year. Although the large motions possible for molecules in the solid state make predicting the course of solid-state dimerization solely on the basis of the nitrile oxide molecular packing arrangements unreliable, we will attempt to prepare new polymorphs of these nitrile oxides to find out whether the particular nitrile oxide packing arrangement can determine the chemical identity of the dimer formed.
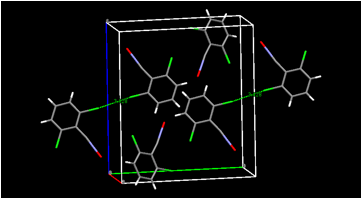
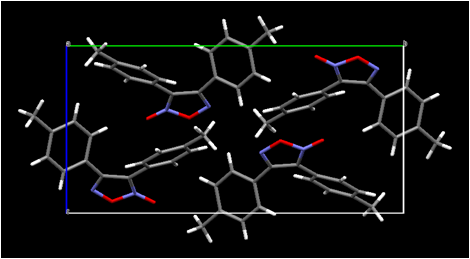
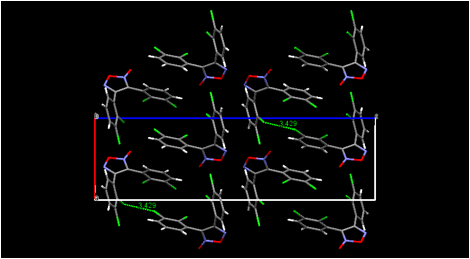
HalogenÉhalogen contacts also are found in the crystal structure of our dichloro-substitued furoxan (Figure 4). Both of our new furoxan crystal structures show evidence of disorder (not shown in the figure) corresponding to a rotation of the molecule about an axis through the ring oxygen atom and the midpoint of the ring carbon-carbon bond. This effectively lends partial occupancy to two sites for the exocyclic oxygen atom, each adjacent to a ring nitrogen atom. This disorder is commonly observed in published furoxan structures (whether actually modeled or not). Disorder of another type apparently is present in all three of our dioxadiazine structures, where it may be a combination of substitutional disorder of nitrogen atoms vs. oxygen atoms and positional disorder of two non-planar ring orientations. We are currently working to model this disorder so that reasonable structures can be published. The fact that these structures are not yet completely refined (although the R factors are approximately 4-6 percent even at this unfinished stage) is the reason for their not being actually shown in this report. Their infrared spectra will help us identify the products of solid-state dimerization.
Figure 3. Selected intermolecular contacts in 3-bromobenzonitrile oxide (top),
2,6-dichlorobenzonitrile oxide (center), and 2,3-dichlorobenzonitrile oxide (bottom).
Figure 4. Views of the molecular packing in bis(4-methylphenyl)furoxan (upper) and in bis(2,3-dichlorophenylfuroxan) (lower). Selected intermolecular halogenÉhalogen contacts are shown for bis(2,3-dichlorophenyl)furoxan.
Although all of our crystal structures are potentially of publication quality, we prefer to publish them only after we have fully characterized the solid-state chemistry of these compounds so that we can place our structures in their proper context and as part of a comprehensive research investigation rather than as isolated crystal structures. We are nevertheless disseminating the work we have done thus far; with ACS-PRF support four of my undergraduate research students presented posters on their work at the 249th ACS National Meeting and Exposition, which was held March 22-26, 2015, in Denver, Colorado. I hope to present a poster on their work at the Spring 2016 Gordon Research Conference on Crystal Engineering. In both cases our posters will reflect the additional progress we intend to make on this project through the coming academic year.