Reports: DNI754298-DNI7: Mechanistic Study on Synthesis of Hyperbranched Polymer with Uniform Structure
Haifeng Gao, University of Notre Dame
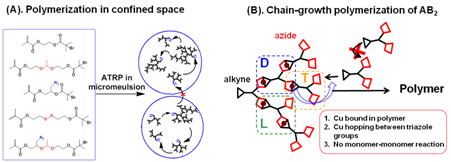
Figure 1. Synthesis of hyperbranched polymers by (A) polymerization in confined space, and (B) chain-growth polymerization of AB2 monomers in solution.
1. Using confined space to produce hyperbranched polymers and hyperstar polymers
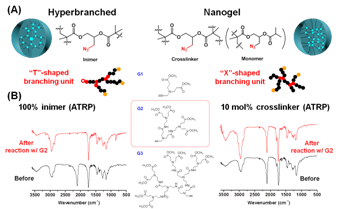
The first approach synthesizes hyperbranched polymers with high molecular weight and narrow molecular weight distribution using confined nanospace, e.g., micelles, to regulate the polymer-polymer coupling reactions (Figure 1A). The key concept in this new method is to segregate the polymerization of reactive monomers into discrete micelles, so that the monomers inside each micelle or polymerizing locus can react/polymerize with each other completely, while there is no inter-micelle reaction. In the past year, the PI's group systematically investigated this novel synthetic method by exploring experimental variables, including 5 methacrylate-based inimer species, 2 ATRP ligands, varied amounts of inimers and catalysts. The new method exhibited great versatility in the polymerization of different inimers and produced a series of hyperbranched polymers with varied compositions, degree of branching (DB = 0.26 - 0.41), molecular weights (Mn = 194 Figure 2. (A) structural difference between hyperbranched polymers and crosslinked nanogels; (B) IR spectra of the hyperbranched polymers and nanogels before and after CuAAC reaction with alkyne-containing G2 dendron.
The obtained hyperbranched polymers that contain T''-shaped branching units, from which three chains radiate out, exhibit superior loading efficiency of guest molecules as compared to crosslinked nanogels with X''-shaped branching units and four extending chains. In Cu-catalyzed azide-alkyne cycloaddition (CuAAC) reactions, azido-containing hyperbranched polymers with similar size and azido group density loaded 3 times more alkynyl-containing dendron molecules than crosslinked nanogels, although the crosslinked nanogels had lower density of branching (10% versus 100%, Figure 2). At the same time, all hyperbranched polymers produced via the polymerization of inimers in microemulsion contain 102-103 bromide initiating sites and could be subsequently used as macroinitiators for polymerization of functional monomers to synthesize hyperstar polymers. This type of synthesis was recently accomplished in the PI's group in a one-pot synthesis via sequential polymerization of inimers and functional monomers in an oil-in-water biphasic media with no star-star coupling. The progress in the use of confined space to regulate polymer architectures has generated three publications in Macromolecules, Polymer Chemistry and Macromolecular Rapid Communications.
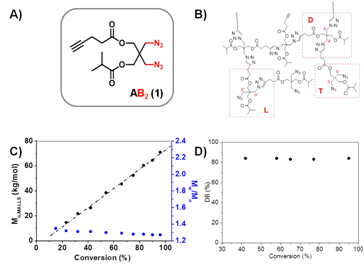
2. One-pot chain-growth polymerization of AB2 monomers in solution The second method presents a one-pot one-batch solution polymerization of AB2 monomer, e.g., AB2 1 in Figure 3A, to produce hyperbranched polymers with high molecular weight (Mn ~ 240 ´103), high DB = 0.83 and low polydispersity (Mw/Mn < 1.3) using CuAAC polymerization (Figure 1B). Complexation of CuI to the in-situ formed triazole groups binds all CuI catalysts in the polytriazole cores at low monomer conversion, and causes subsequent CuAAC reactions only occurring around the polymer proximity between polymers and monomers. The reactions exhibited linear increase of molecular weight versus conversion and clean chain extension in multiple batches of monomer addition, demonstrating key feature of chain-growth polymerization mechanism (Figure 3C). Meanwhile, the complex of CuI/triazole activated fast reaction of the 2nd azide group and quickly converted the linear (L) unit to dendritic (D) unit, producing hyperbranched polymers with high DB = 0.83 (Figures 3C and 3D).
Figure 3. (A) AB2 1 monomer; (B) representative structure of hyperbranched polymer; evolution of (C) absolute molecular weight (Mn,MALLS), polydispersity (Mw/Mn); and (D) degree of branching (DB) as a function of monomer conversions of hyperbranched polymers synthesized using: [AB2 1]0:[CuSO4]0: [ascorbic acid]0 = 100:1:5, in DMF at 45 °C.
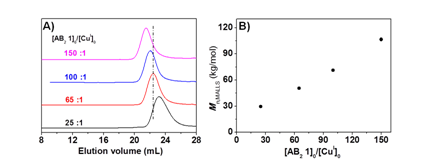
Under constant concentration of [AB2 1]0 = 0.5 mol/L, varied ratios of [AB2 1]0/[CuI]0 = 25-150 demonstrated a linear increase of polymer molecular weight at complete conversion as the amount of Cu catalyst decreased (Figure 4), indicating that less Cu catalyst decreased the molar concentration of polytriazole cores formed at the initial stage and resulted in hyperbranched polymers with higher molecular weights.
The results in the progress have been recently published in Angew. Chem., Int. Ed. with more results being submitted to high-quality journals. Considering the broad application of CuAAC reaction in polymer synthesis, the easy functionalization of AB2 monomers, and the potential coordination of triazole with metal cations, this one-pot synthetic method of chain-growth CuAAC polymerization is expected to generate broad interest in both polymer chemistry and materials science.
Figure 4. (A). THF SEC traces; (B). Mn,MALLS of hyperbranched polymers at various ratios of [AB2 1]0:[CuSO4]0: [ascorbic acid]0 = X:1:5 in DMF at 45 °C, X = 25, 65, 100 and 150, [AB2 1]0 = 0.5 mol/L.
3. Educational impact on training students and postdoc The research progress has generated three significant educational impacts on training students and postdoc researcher. First, the proposed experiments and research achievements allows the participating graduate student, postdoc and undergraduate student (registering for research credit with no stipend) to learn the state-of-the-art polymer chemistry knowledge, experimental skills and get hands-on experience on various polymer characterization techniques. Second, the PI has organized two annual Notre Dame soft materials symposia since 2014 and the graduate student and postdoc got opportunity to present their research in front of 70 symposium participations. Third, the research results have been integrated in the PI's polymer chemistry course to senior undergraduate students and graduate students from both Department of Chemistry and various Engineering departments.