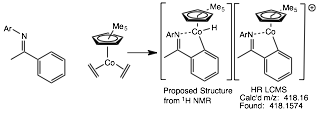
Reports: UR351716-UR3: Ligands for the Cobalt-Catalyzed Dimerization of Alpha Olefins
Richard D. Broene, Bowdoin College
Tethered Ligands
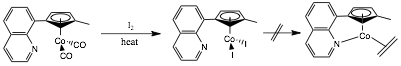
We synthesized the complex diiodo-η5-[1-(8-quinolyl)-3-methylcyclopentadiene]-cobalt(III)
with the idea that a Cp ring less donating than Cp*
would facilitate reduction to Co(I). Scheme 1. CH Activation.
We began a series of studies to understand if Co(I) species
could activate CH bonds.