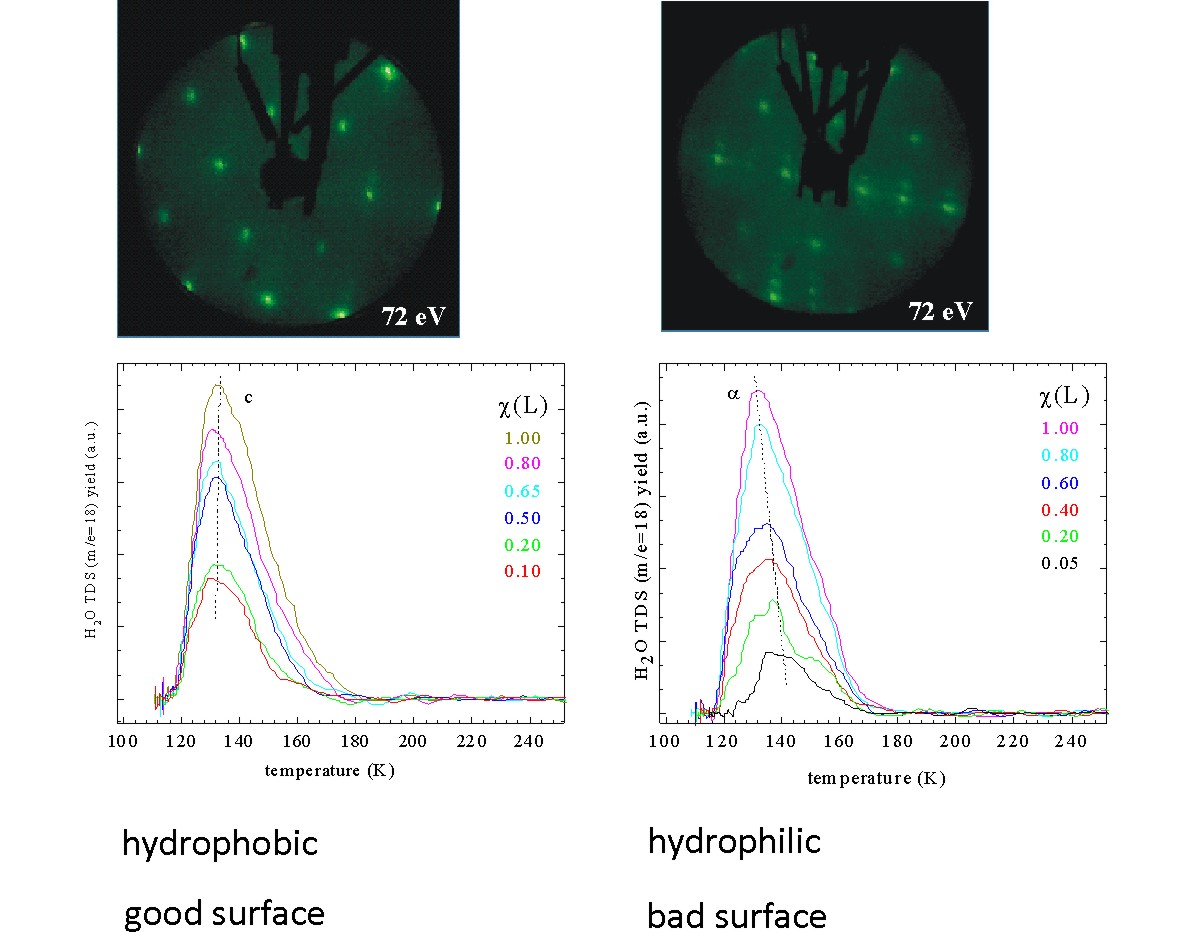
Reports: ND554179-ND5: Fundamental Properties of Two-Dimensional Zeolites
Uwe Burghaus, North Dakota State University
1) Synthesize/fabricate 2D zeolite-like films (“Al-doped silica films”), which
requires first to synthesize 2D crystalline silica; 2) Use thiophene
to probe catalytic activity by means of surface chemistry techniques; 3)
Deposit Mo on/in the porous support targeting hydrodesulphurization–related
(HDS) surface chemistry as petroleum-related research.