Reports: ND753960-ND7: Well-Defined Polyrings via Diyne-Functionalized Cyclic Monomers
Chong Cheng, PhD, State University of New York at Buffalo
The objective of this research project is to establish a novel synthetic approach for the preparation of well-defined polyrings, which are polymers with each repeat unit carrying a sizable ring without a spacer. Polyrings can be considered as ideal model polymers to probe the influence of sizable side rings on polymer properties, but the synthetic approach for such polymers has not been established. Accordingly, the hypothesis of this project is that well-defined polyrings can be synthesized by metathesis polymerization of cyclic derivatives of diynes. To examine this hypothesis, cyclic diyne monomers need to be prepared at first, and then their metathesis polymerization should be studied and optimized, in order to obtain well-defined polyrings. Significant research efforts have been made to investigate synthetic routes for the preparation of series of cyclic diyne monomers.
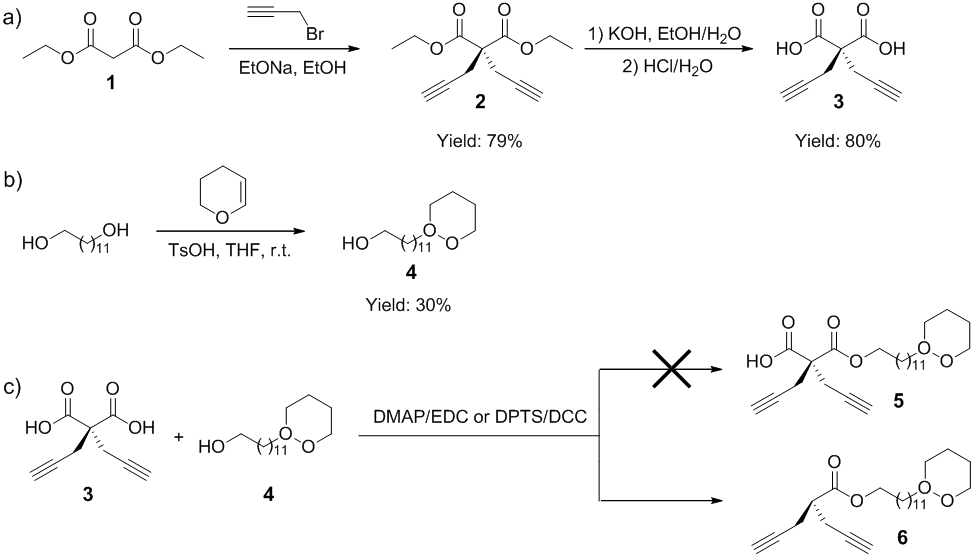
We studied the synthetic route shown in Scheme 1 at first. Following a literature approach,1 diethylmalonate (1) was reacted with propargyl bromide in the presence of sodium ethoxide in dry ethanol at reflux for overnight, and diethyldipropargyl malonate (2) was obtained in 79% yield (Scheme 1a). Then hydrolysis of 2 catalyzed by potassium hydroxide in the water/ethanol gave diethyldipropargyl malonic acid (3) in 80% yield. The esterification reaction between diacid 3 and diol was attempted, but the results indicated difficulties in synthetic control to suppress the formation of oligomers as side products. Therefore, mono-protection of 1,12-dodecanediol, a representative diol, was performed. Following literature approach,2 1,12-dodecanediol was treated with dihydropyran (DHP) in the presence of tosylic acid (TsOH) in dry THF at room temperature for overnight, yielding the mono-protected diacid (4) in 30% yield (Scheme 1b). The low yield of 4 was ascribed to the difficulty in separation of 4 from the reaction mixture. The esterification reaction of 3 and 4 was then investigated by using different catalytic systems. Unfortunately, the targeted mono-acid dipropargyl esterification product (5) could not be obtained at all. Both 1H NMR analysis and high resolution mass spectroscopy (HRMS) indicated the occurrence of decarboxylation along with the esterification reaction, and the resulting product (6) had no remaining carboxyl group. Along with this experimental finding, a reference reporting the decarboxylation of malonic acid under mild conditions was also found.3 Thus, the initial plan of synthesis of cyclic diyne monomer via 5 by deprotection of DHP-protected hydroxyl group followed by ring-closing unimolecular esterification reaction could not succeed, and the synthetic route must be revised.
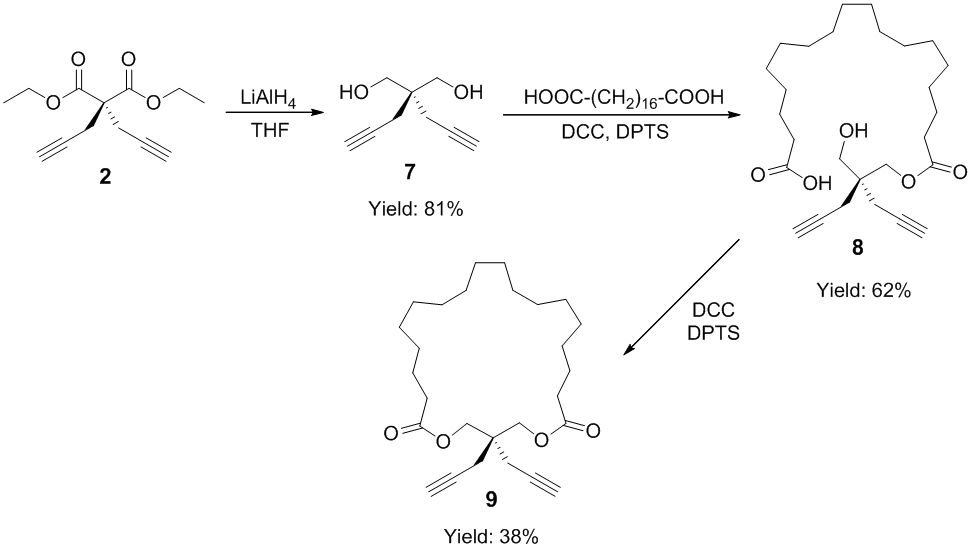
Subsequently, Scheme 2 was utilized for the synthesis of cyclic diyne monomers. Following a literature approach,1 compound 2 was treated with LiAlH4 for overnight in THF at room temperature, and 4,4-bis(hydroxymethyl)-1,6-heptadiyne (7) was obtained in 81% yield. Then catalyzed by N,N'-dicyclohexyl-carbodiimide (DCC) and 4-(dimethyleamino)pyridinium 4-toluene-sulfonate (DPTS), octadecanedioic acid was reacted with an excess of 7 (5 eq), giving 18-[(2-(hydroxymethyl)-2-(prop-2-ynyl)pent-4-ynyl)oxy]-18-oxooctadecanoic acid (8) in 62% yield. Finally, the corresponding cyclic diyne monomer (9) was obtained in 38% yield by unimolecular ring-closing esterification reaction of 8 catalyzed by DCC/DPTS in dichloromethane. With a successful synthetic trial in the preparation of 9, currently we are studying the reproducibility of this synthesis and we are also preparing other cyclic diyne monomers with different sizes of the alkyl-based rings.
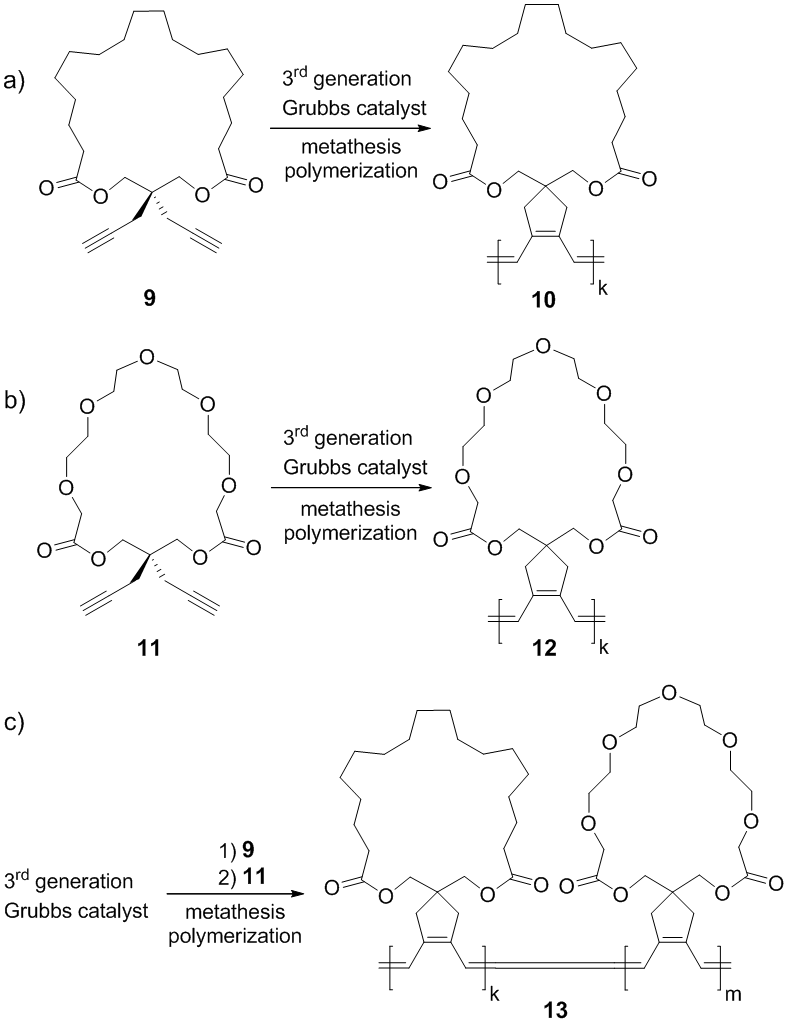
In summary, synthetic routes for the preparation of cyclic diyne monomers were investigated in the first grant year. The synthetic route via diethyldipropargyl malonic acid was excluded due to the serious decarboxylation side reaction during the esterification step, and on the other hand, the synthetic route via a diacid-functionalized heptadiyne was verified as a valid route to generate cyclic diyne monomers. In the next grant year, we plan to 1) study metathesis polymerization of cyclic monomer 9 and other diyne monomers with alkyl-based rings (Scheme 3a), 2) synthesize cyclic diyne monomers with oligo(ethylene glycol)-based rings and study metathesis polymerization of these monomers (Scheme 3b), and 3) study the sequential metathesis polymerization of different types of cyclic diyne monomers to obtain diblock polyrings (Scheme 3c).
References:
1) Xu, L. Q.; Yao, F.; Fu, G. D.; Shen, L. Macromolecules, 2009, 42, 6385.
2) Zhang, Q.; Ren, H.; Baker, G. L. Tetrahedron Letters 2014, 55, 3384.
3) Lafrance, D.; Bowles, P.; Leeman, K.; Rafka, R. Organic Letters, 2011, 13, 2322.
Scheme 1
Scheme 2
Scheme 3