Reports: UR351747-UR3: Hydrogen Production via Homogeneous Catalysis: Investigations of the Water-Gas-Shift Reaction
Jason S. D'Acchioli, PhD, University of Wisconsin (Stevens Point)
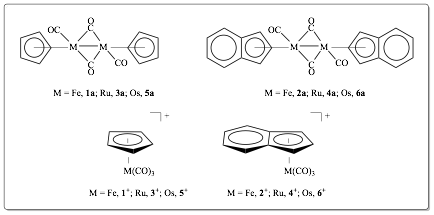
Figure 1. Target complexes under investigation.
Figure 2. The water-gas-shift reaction.
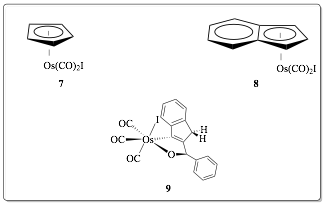
2. Progress. Our attention over the past year was heavily focused on optimizing syntheses of complexes 7 and 8 (which are fairly easily accessible analogues of 5+ and 6+, respectively). Complex 7 was characterized by 1H, 13C, and IR spectroscopies, as well as single-crystal X-ray diffraction studies; complex 7 was characterized as described in last year's report. We have improved our yields of 7 from 7% to 48%, and of 8 from 7% to 27%. The source of our improved yield is due to immediate workup of the reaction mixtures. During the academic year, students typically place finished reactions in a 5°C freezer for a period of two or three days prior to workup; this is a function of their class schedules. Summer, however, permitted students to work up reactions immediately; their yields in each case were dramatically improved. We reported 9—a byproduct in the formation of 8—in last year's PRF report. We explored its production by a) excluding light from the reactions, and b) varying solvents from benzene, to toluene, to o-xylene, to mesitylene. The light-excluded reactions in the aforementioned solvents led to extremely low yields—if any at all—of product (ca. 3.6 %). The reactions performed with light have led to mixtures of 8 and 9, although immediate workup in toluene seems to stem the formation of 9. Due to the expense of the Os3(CO)12starting material ($495 for a 2 gram vial from Strem Chemicals; each reaction consumes 0.5 g), however, we are limiting our mechanistic explorations.
Figure 3. Complexes considered in this report. Our work during the 2015 summer concluded with experiments testing the efficacy of 7 and 8 as WGS reagents, via reaction with wet triethylamine (reaction conditions are similar to those in Reference 1). We are in the process of analyzing those results.
3. Impact on PI. The PRF grant has had a significant impact on the PI's career. First, it has allowed me to retain two outstanding undergraduate students. Second, it has allowed me to maintain my fruitful collaboration with Professor Arnold Rheingold (UCSD), who is determining structures through single-crystal diffraction, the most recent being compound 7.
4. Impact on Undergraduates. The students working on this project are two female undergraduates continuing their work on this project. Both students presented results from their 2013-2014 academic year work at the ACS National Meeting in Denver, Colorado, in 2015. Their presentation was a part of the of the "Undergraduates at the Frontiers of Inorganic Chemistry" poster session. Both students' results from summer 2015 are being compiled into a manuscript, which we intend to submit for publication by the beginning of 2016. 5.
References.
1. Badger, R. C.; D’Acchioli, J. S.; Oudenhoven, T. A.; Walder, B. J. Organometallics. 2010, 29, 1061-1063.
2. Rosenberg, S.; Herlinger, A. W.; Mahoney, W. S.; Geoffroy, G. L.; Hembre, R. T.; Birdwhistell, K. R.; Norton, J. Inorg. Synth. 1989, 25, 187-192.
3. Sun, S.; Dullaghan, C. A.; Carpenter, G. B.; Rieger, A. L.; Rieger, P. H.; Sweigart, D. A. Angew. Chem. Int. Ed. 1995, 34, 2540-2542.