Reports: DNI552743-DNI5: Gas-Phase UV Grafting on Surface-Initiated ROMP Coatings for Improved Stability
Bradley J. Berron, PhD, University of Kentucky
This effort is focused on the investigation of the chemistry, structuring and stability of coatings formed from surface-initiated ring-opening metathesis polymerization (SI-ROMP) coatings. We focused this investigation on two distinct areas: 1) the study of the stability of the underlying self-assembled monolayer, and 2) the study of solvent-induced degradation of the SI-ROMP coatings. In each we explored the role of the proposed thiol-grafting in the stability of these coatings.
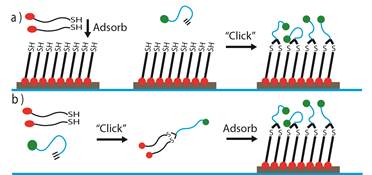
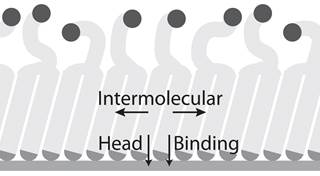
Improving the stability of the self-assembled monolayer adsorbate. In our study of the nature of monolayer-gold bonding, we observed poor solvent and electrochemical stability of the base monolayer used to grow the ROMP coatings. Specifically, the solvents used in SI-ROMP would lower the crystallinity of the alkyl region of the monolayer, and reducing the component of monolayer stability associated with intra-chain stabilization. We proceeded with a hypothesis that a y-shaped adsorbate with two gold-interfacing thiol bonds would provide a more stable monolayer and an environment-interfacing group with the conformational freedom required for further functionalization and polymerization. Adsorbates are synthesized through the thiol-yne addition of two thiol-containing head groups to an alkyne-containing tail group (Figure 1b). The resulting monolayers have two distinct phases: a highly crystalline head phase adjacent to the gold substrate, and a reduced density tail phase, which is in contact with the environment (Figure 1). The ellipsometric thickness of 27 Å is consistent with the proposed structure, where a densely packed decanedithiol monolayer is capped with an 11 carbon long, second layer at 50% lateral chain density. The Fourier transform infrared peaks associated with asymmetric and symmetric methylene stretching are shifted toward higher wavenumbers compared to those of well-packed self-assembled monolayers (SAMs), which shows a lower average crystallinity of the thiol-yne monolayers compared to a typical monolayer. Contact angle measurements indicate an intermediate surface energy for the thiol-yne monolayer surface, owing to the contribution of exposed methylene functionality at the surface in addition to the acid terminal group. The conformational freedom at the surface was demonstrated through remodeling the thiol-yne surface under an applied potential. Changes in the receding contact angle in response to an external potential support the capacity for reorientation of the surface presenting groups. Despite the low packing at the solution interface, thiol-yne monolayers are resistant to water and ion transport, supporting the presence of a densely structured layer at the gold surface. Further, the electrochemical stability of the thiol-yne adsorbates exceeded that of well-packed SAMs, requiring a more reductive potential to desorb the thiol-yne monolayers from the gold surface. The thiol-yne monolayer approach is not limited to carboxylate functionality and is readily adapted for low-density monolayers of varied functionality. This work is published as an article in Langmuir.
Figure 1. The design of thiol-yne adsorbates for low interfacial density and high monolayer stability. (a) Solid phase synthesis of a bifunctional adsorbate through dithiol adsorption and subsequent click-chemistry. (b) Solution phase synthesis of a bifunctional adsorbate through click-chemistry and subsequent adsorption. (right) Description of stabilizing forces in thiol-yne SAMs.
We have continued in this promising area of surface chemistry with the simplified synthesis of these highly stable thiol-yne adsorbates through a surface grafting approach (Figure 1a). The resulting monolayers are compared with a well-packed 11-mercaptoundecanoic acid monolayer and the analogous low-density monolayers prepared through a solution phase synthetic approach. The overall structuring of the monolayer prepared by solid-phase grafting is characterized by contact angle goniometry and Fourier transform infrared spectroscopy. The results show that the product monolayer has an intermediate surface energy and a more disordered chemical structuring compared to a traditional well-packed self-assembled monolayer, showing a low-packing density of the chains at the monolayer surface. The monolayer’s structure and electrochemical stability were studied by reductive desorption of the thiolates. The prepared low-density monolayers have a higher electrochemical stability than traditional well-packed monolayers, which results from the crystalline structure at the gold interface. This technique allows for simple, fast preparation of low-density monolayers of higher stability than well-packed monolayers. The use of a photomask to restrict light access to the substrate yielded these low-density monolayers in patterned regions defined by light exposure. This general thiol-yne approach is adaptable to a variety of analogous low-density monolayers with diverse chemical functionalities. This work is published as a second article in Langmuir.
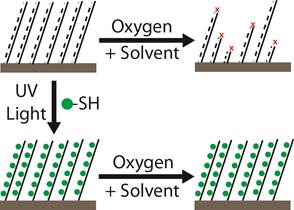
Stability of SI-ROMP. Here, we investigate the grafting of thiols to surface-initiated polynorbornene (pNB) as a novel approach towards highly-stable, ROMP-type conformal coatings (Figure 2). We have developed the grafting reaction in the solution phase, where alkyl or fluorocarbon thiols are grafted to the pNB backbone at olefinic sites. Conversion of olefin groups is >95% by FTIR, and the resulting FTIR spectra have appropriate additional peaks for linear alkyl or fluorocarbon groups. The removal of the olefin functionality from the polymer backbone decreased the rate of coating damage from solvents by over 50%. As expected, the coatings are still damaged by solvents, as the removal of olefin is incomplete, and 100% conversion would be required for complete protection by thiol-ene grafting.
Figure 2. Surface-initiated ROMP polymers are unstable in oxygenated solvent. Gas-phase grafting of thiols to the unsaturated backbone improves polymer stability by consuming alkene functionality.
Based on these findings, we are now evaluating the influence of crosslinking on the stability of SI-ROMP coatings. Our initial findings indicate a significant decrease in the rate of solvent erosion with the use of a custom synthesized PEG dinorbornene (diNB, Mn ~3400). Additionally, the diNB alters the kinetics of SI-ROMP. The presence of the functionlized norbonene molecule decreases the overall polymerization rate constant. Additionally, the polymerization takes on a more living character. While SI ROMP of norbornene reaches a terminal thickness after several minutes, films grown with 0.25 mol% diNB in norbornene grow steadily over 2 hours. This sustained growth is preliminarily attributed to prolonged attachment of the catalyst on the surface, and follow up studies are underway to evaluate this hypothesis. We expect to publish these findings as a full article in Macromolecules.