Reports: ND153385-ND1: Borenium Catalysts for Metal-Free Enantioselective Reductions and Petasis Reactions
Cathleen M. Crudden, Queen's University
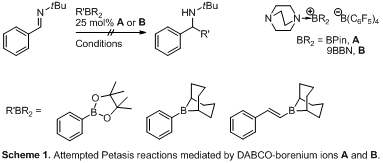
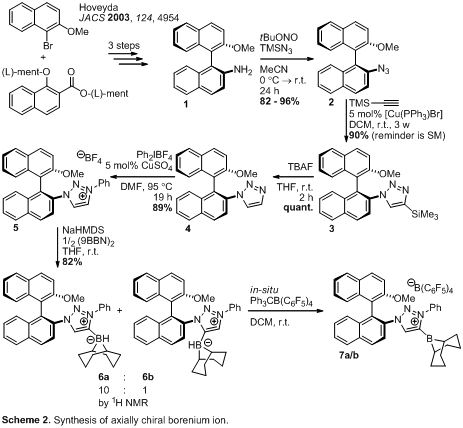
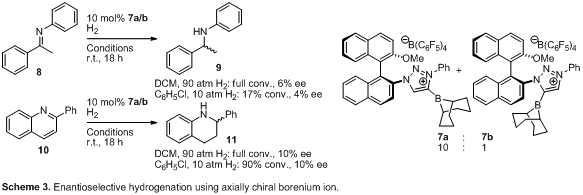
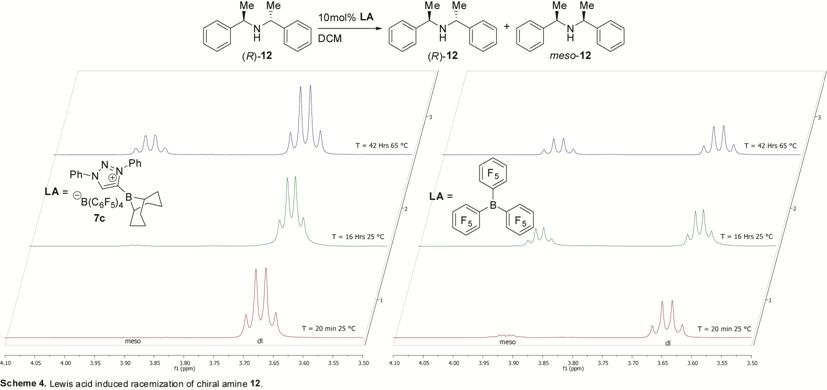
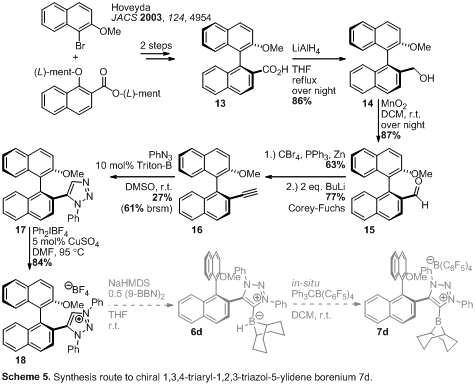
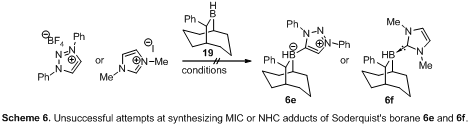
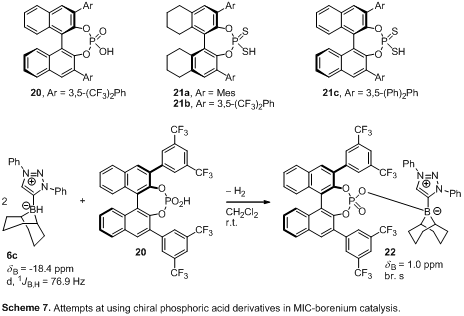
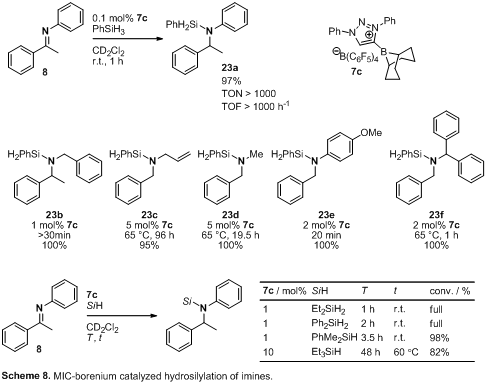
Cathleen M. Crudden, Queen's University








Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society