Reports: DNI153703-DNI1: Cobalt-Catalyzed Carbon-Carbon Bond Forming Reactions of Simple Olefins
Thomas J. Maimone, PhD, University of California, Berkeley
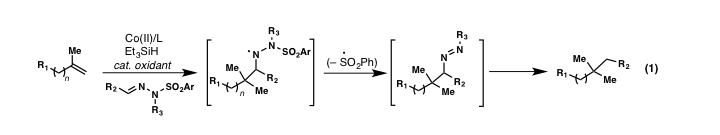
The ability to convert simple unfunctionalized olefins into more complex, higher value chemicals is a significant thrust for modern chemistry and the theme of this research program. Metal-catalyzed olefin hydrofunctionalization, as originally developed by Mukaiyama and Isayama using Cobalt catalysis, has recently witnessed an explosion in growth for the construction of C–X bonds (X = F, Cl, O, H, N, S) from simple alkenes. At the start of our work, however, only a single report detailed useful C–C bond forming processes using this radical-based manifold. Very recently, several new C-C bond new forming processes have begun to emerge. We proposed the development of a novel, Co-catalyzed hydroarylation reaction using simple hydrazones as reagents that can transfer alkyl groups to alkenes in accordance with equation 1.
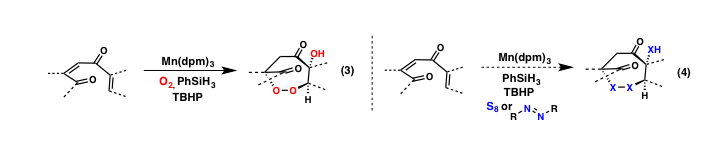
During the past grant period we have prepared and evaluated numerous aldehyde and ketone-derived hydrazones in an effort to indentify suitable alkyl transfer reagents and conditions for this process. We have obtained results indicating this challenging transformation is possible in a single step (equation 2), yet substantial optimization will be required during the final stages of the funding cycle. Notably, during the course of our studies, a report by Baran and co-workers described a highly similar transformation using Fe-catalysis. These studies also highlight the difficulty of a one-pot nitrogen extrusion step, something we are keen on solving during the upcoming grant period.
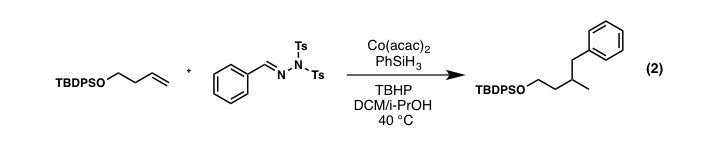 Continuing with our laboratories interest in alkene functionalization chemistry using inexpensive transition metals (Co, Mn, Fe), we have has also recently discovered a novel peroxidation cascade under Mn-catalysis leading to complex, 7-membered endoperoxide scaffolds from simple starting materials (equation 3). During the upcoming grant period, extension of this chemistry toward polyamination and sulfidation (equation 4) will be developed with the ultimate goal of enhancing the toolbox of conditions for the simple and cost-effective functionalization of alkene units.
Continuing with our laboratories interest in alkene functionalization chemistry using inexpensive transition metals (Co, Mn, Fe), we have has also recently discovered a novel peroxidation cascade under Mn-catalysis leading to complex, 7-membered endoperoxide scaffolds from simple starting materials (equation 3). During the upcoming grant period, extension of this chemistry toward polyamination and sulfidation (equation 4) will be developed with the ultimate goal of enhancing the toolbox of conditions for the simple and cost-effective functionalization of alkene units.
We are truly grateful to the ACS PRF for their support of our work, which has had a profound effect on the PI's early career. This support has allowed the PI to substantially broaden his research portfolio as well as train and mentor future synthetic chemists.