Reports: DNI654407-DNI6: Direct Visualization of Interfacial Energy-Transfer and Charge-Transfer Dynamics of Thin Ionic Liquid Films by Ultrafast Electron Imaging
Ding-Shyue Yang, PhD, University of Houston

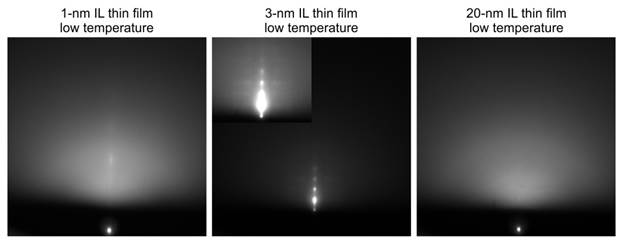
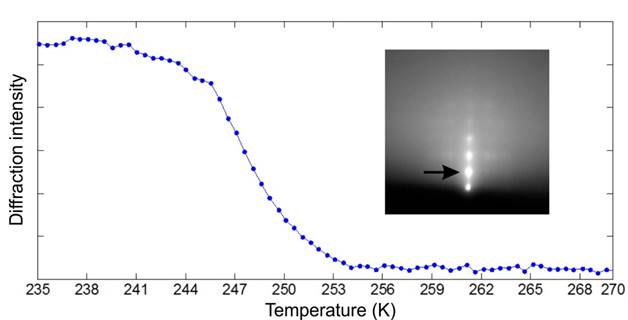
Ding-Shyue Yang, PhD, University of Houston



Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society