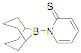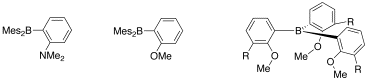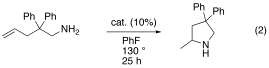Reports: ND352856-ND3: Stabilization of Low-Oxidation State Main Group Complexes with sigma-Acceptor Z-Type Ligands
Rudolf J. Wehmschulte, Dr. rer. nat., Florida Institute of Technology
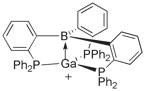
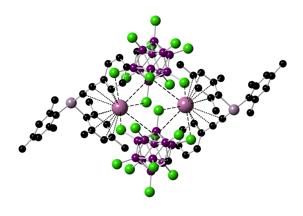
Figure 1. Sketch of an early target compound.
Ligand Synthesis
We have prepared the ligand B(C6H4PPh2)3 and the related mono- and bidentate ligands Mes2BC6H4PPh2 (Mes = 2,4,6-Me3C6H2-) and PhB(C6H4PPh2)2. The synthesis of the more soluble isopropyl analogues is ongoing. The monodentate FLP type ligand cis-Mes2P(Ph)C=C(C6F5)B(C6F5)2 (FLP = frustrated Lewis Pair) is available in our lab, and we have synthesized the mercaptopyridine ligand 9-BBN-(NC5H4S-2) as shown below.
Figure 2. Schematic structure of 9-BBN-(NC5H4S-2).
Reactivity Studies of Ga+ and In+ Compounds
In the previous year, we have been successful in synthesizing the M(I) compounds [M(arene)n][CHB11Cl11] (arene = toluene, bromobenzene) according to eq 1.
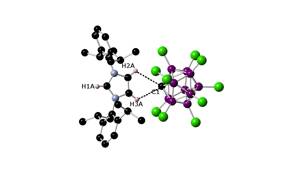
The structures of the indium species [In(toluene)3][CHB11Cl11] and [In(C6H5Br)1.5][CHB11Cl11] were determined, but the structure of the gallium analogues has been elusive to date, although crystalline products have been obtained. While the related indium(I) compound [In(C6H5F)2][Al{OC(CF3)3}4] afforded crystalline complexes with PPh3, PtBu3 and the N-heterocyclic carbene IDipp, our indium(I) arene complexes led to redox reactions affording indium metal and the oxidized ligands [HPPh3]+ and imidazolium [IDippH]+. An excess of carbene resulted in the deprotonation of the carborate anion as shown in Figure 3.
Figure 3. Structure of the tight ion pair [IDippH]+[CB11Cl11]2-.
It is not yet clear why the reactivity of our indium(I) compounds with the carborate counterion is different from those with the alanate counterion. As it indicates that electron rich and oxidizable ligands such as phosphines and carbenes lead to redox reactions, we plan to employ the ligands shown below featuring harder N and O donor centers.
Figure 4. Sketches of ligands with N and O donor centers.
The interactions between In+ and soft ¹-systems including olefins and alkynes is also rather weak, but the employment of the less volatile phosphinoalkyne Mes2PC¼CPh resulted in the isolation of the crystalline adduct [In(PhC¼CPMes2)][CHB11Cl11], whose structure consists of two [In(PhC¼CPMes2)]+ units bridged by two anions. Interestingly, the In+ centers are coordinated by the ¹-systems of the triple bond and one of the mesityl rings and several chlorines of the anion and not by the phosphorus donor. In fact, the phosphorus lone pair points away from the indium centers.
Figure 5. Crystal structure of [In(PhC¼CPMes2)][CHB11Cl11].
Compound [In(C6H5Br)1.5][CHB11Cl11] catalyzed the intramolecular hydroamination of aminopentenes affording substituted pyrollidines.
However, at present the identity of the catalytically active species is not clear. The formation of a small amount of a fine black solid (presumably In metal) during the reaction indicates redox activities, so that the active species could be an indium(III) species or even a Brönsted acid.
Anion Synthesis
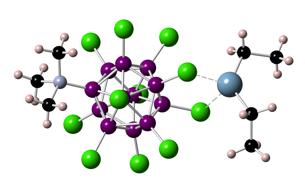
The isolation of arene solvated gallium(I) and indium(I) cations requires the use of weakly coordinating anions such as the carborate anion [CHB11Cl11]- used in this study. Unfortunately, the synthesis of this anion is lengthy and requires the expensive and toxic precursor decaborane(14), B10H14. We have been able to optimize the chlorination reaction of the expensive precursor [CH12B11]- with SO2Cl2 by UV irradiation. Furthermore, we have synthesized the zwitterion [Me3NB12Cl11]-, which uses the readily available [B12H12]2- starting material. Although this anion forms the ion pair [Et2Al[Me3NB12Cl11] in good yields, its silver salt is only slightly soluble in bromo- or fluorobenzene, making it impractical as counterion for our gallium(I) and indium(I) cations.
Figure 6. Crystal structure of [Et2Al[Me3NB12Cl11]