Reports: ND451880-ND4: The Selective and Sequential Chemistry of Electronically-Coupled Lactones
Ronald K. Castellano, University of Florida
A. Background and significance.
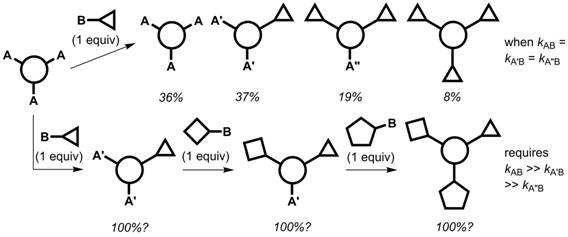
Synthetic methodology to rapidly and efficiently prepare multifunctional molecules continues to leverage discoveries in disciplines spanning materials science and chemical biology. One approach to discrete multifunctional molecules is illustrated in Figure 1. A statistical mixture (with the percentages shown) results if the reaction rates between reagent site B and positions A, A′, and A″ are identical (top pathway). If each successive functionalization reaction sufficiently deactivates the remaining reactive sites, and a reactivity gradient is established, a one-pot sequential multifunctionalization can be executed (bottom pathway).
Figure 1. The kinetics
associated with statistical and non-statistical syntheses of discrete
multifunctional molecules from a three-fold symmetrical precursor.
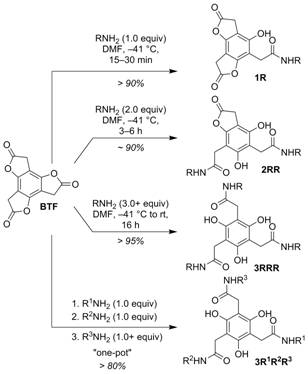
We recently reported that benzotrifuranone
(BTF) (Scheme 1) is uniquely suited for multifunctionalization
through sequential aminolysis reactions. The
platform affords rapid access to mono- (1), di-
(2), and trifunctionalized (3) targets
provided routine control of temperature and amine reagent stoichiometry,
and lends itself to the one-pot synthesis of multifunctionalized
3 (i.e., 3R1R2R3) from 1
in high yield (> 80%) and a single day. During the last two funding
periods we obtained a more comprehensive understanding of the aminolysis behavior of BTF through comparative
kinetics measurements, X-ray crystal structure analysis, and quantum chemical
calculations. Particularly useful for the studies was BTF’s six-membered lactone congener, benzotripyranone (BTP, Table 1). Data from the
studies, the basis for a nearly complete full paper, pointed strongly to “ring
strain” as a significant reactivity driver for BTF. Work this past
year has sought to (a) validate the ring strain hypothesis through additional
quantum chemical calculations and (b) apply the multifunctionalization
capabilities of 1 to the synthesis of a molecular dye that functions
through an energy transfer cascade.
Figure 2. Aminolysis of benzotrifuranone (BTF). B. A Ring Strain Gradient as a Sequential Aminolysis Selectivity Driver. During this last reporting period we performed a series of
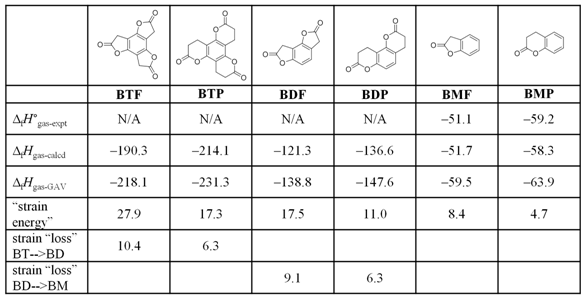
theoretical (DFT; B3LYP/6-31+G**) calculations to estimate the “strain energy”
of the suite of compounds shown in Table 1. The model heterocycles
represent substructures of the species formed along the aminolysis
pathways of both BTF and BTP. To evaluate strain, theoretical
heats of formation at 298 K (ΔfHgas-calcd)
—determined from appropriate homodesmotic equations
involving experimental ΔfHgas
values—were compared to values determined for hypothetical “unstrained”
molecules (through strain-free group increments). The trends in strain
energy values are consistent with the overall aminolysis
rate behavior found for the individual BTF and BTP series.
That is, kBTF > kBDF > kBTP
> kBDP (on a per lactone basis) as strain (on a per lactone
basis) decreases as BTF > BDF > BTP > BDP.
Moreover, the difference in BT-->BD strain loss between BTF
and BTP (10.4–6.3 = 4.1 kcal mol-1) is in the ballpark of the
activation energy difference (~ 3 kcal mol-1) that corresponds to
the 125-fold difference in experimental monoaminolysis
rate between the compounds. More interesting is that the change in ring
strain along BTF-->BDF-->BMF is not constant; the
strain loss (10.4 kcal mol-1) is greater in the first step than the
second (9.1 kcal mol-1). Such is not the case for BTP-->BDP-->BMP
where the same magnitude of strain release (6.3 kcal mol-1) is
found for both steps. The “gradient” observed in the BTF series is
a novel observation among reactive small-molecule systems and helps to
rationalize the unusual aminolysis reactivity of the
parent molecule.
Table 1. Theoretically
determined heats of formation (gas phase, 298 K) and associated strain energies
for the heterocycles involved in the sequential aminolysis of BTF and BTP. The strain trends parallel experimental aminolysis
rate trends described previously and support the hypothesis that strain release
is an important driver of aminolysis selectivity in
the systems. All values are in kcal mol-1. GAV =
strain-free group additivity value.
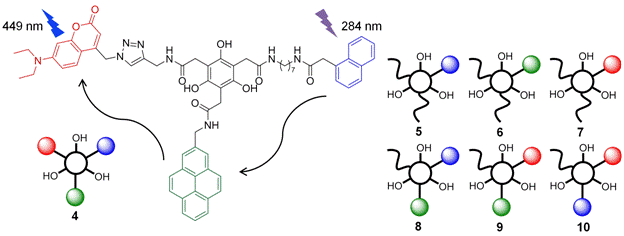
In a
separate line of investigation (summarized in Figure 3) we are using the BTF
multifunctionalization chemistry to prepare unique
dye “cassettes” that function through an internal energy transfer (FRET)
cascade. The target is shown as 4 and borrows a family of dyes
originally reported in 2013 by Das and co-workers: a naphthalene (FRET donor
#1), a pyrene (FRET acceptor #1 and donor #2), and a coumarin (FRET acceptor #2). To date, all of the
possible mono- and dichromophore control compounds
have been prepared (5–10) and fully analyzed by UV-Vis absorption
and fluorescence emission spectroscopy in CHCl3. The coumarin is attached via the “click” chemistry described in
the previous annual report. Quantum yield determinations have provided a
way to quantify the energy transfer efficiency in the dyads, which is ~ 95% in 8
and 9 (consistent with their designed FRET pairs), and a lower ~ 68% in 10
(donor #1 to acceptor #2). We anticipate a
correspondingly high energy transfer efficiency (~ 90%) in 4
wherein excitation at 284 nm will produce emission at 449 nm (for an effective
Stokes shift of 165 nm). Unique about the cassettes (versus ones
potentially derived from cyanuric chloride) is the
opportunity for their attachment to other organic molecules through the free phenolic (OH) positions.
Figure 3. A FRET cassette (4)
available through sequential multifunctionalization
of BTF; the superdye functions through an
internal energy transfer cascade. Model compounds 5–10 have
been prepared and evaluated spectroscopically during
the reporting period.
D. Final Remarks. Synthetic multifunctionalization
strategies are critical for realizing sophisticated properties from molecules
sufficient for their broad applications in the biological and materials
sciences. The chemistry described above, performed by several graduate
students and one undergraduate, is working towards this goal. The grant
has had an enormous impact on the students involved in the project, particularly
with respect to their hands-on training in the chemical sciences.