Reports: ND554684-ND5: Spectro-Electrochemical Microscopy of Single Nanoparticle Electrocatalysts
Christy F. Landes, PhD, Rice University
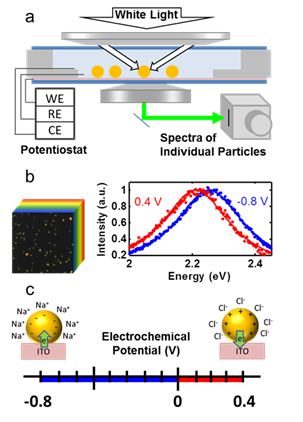
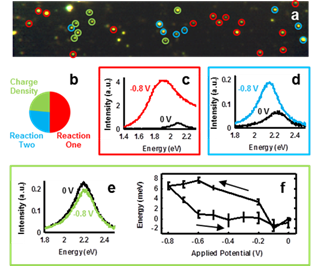
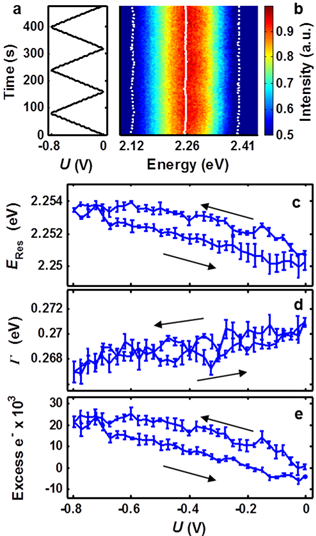
Christy F. Landes, PhD, Rice University



Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society