Reports: ND753225-ND7: Accelerated Preparation of Polymer-Stabilized Catalytic Nanoparticles
Brent S. Sumerlin, PhD, University of Florida
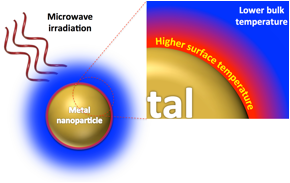
Figure 1. Selective superheating of gold nanoparticles
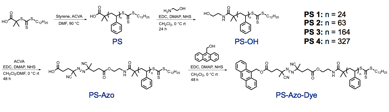
Polystyrene was functionalized with ethanolamine (EA), a thermally labile azo initiator, 4,4′-azobis(4-cyanovaleric acid) (ACVA), and fluorescent reporter molecule, 9-anthracenemethanol (9AM) (Table 1, Scheme 1).
Table 1. Polymeric spacers PS 1-4
Entry |
DPn |
Mn (g mol-1) |
Mw (g mol-1) |
Mw/Mn |
Rg (nm) |
PS 1 |
24 |
2 500 |
2 600 |
1.05 |
0.51 |
PS 2 |
63 |
6 600 |
6 700 |
1.01 |
0.83 |
PS 3 |
164 |
17 100 |
17 800 |
1.04 |
1.34 |
PS 4 |
327 |
32 100 |
34 400 |
1.07 |
1.87 |
Scheme 1. Synthesis and functionalization of polymeric spacers
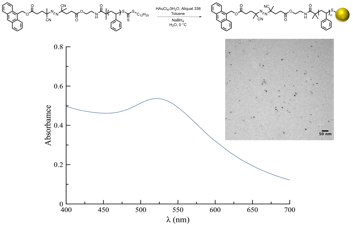
A modified two-phase AuNP synthesis was carried out at 0 °C, yielding stabilized thiolate-protected AuNPs (D ~ 4-6 nm, Figure 2).
Figure 2. Synthesis, UV-Vis spectrum and Transmission Electron Microscopy micrograph of gold nanoparticles
The grafting densities of polymeric ligands on the various AuNPs were measured using TGA. The properties of AuNP samples 1-4 are summarized in Table 2.
Table 2. Properties of AuNP 1-4
Entrya |
Polymer Mw (g mol-1) |
Davg (nm) |
Ligand Density (chains nm-2) |
AuNP 1 |
2 600 |
5.7 ± 0.9 |
0.85 |
AuNP 2 |
6 700 |
5.6 ± 0.6 |
0.28 |
AuNP 3 |
17 800 |
5.7 ± 1.1 |
0.29 |
AuNP 4 |
34 400 |
6.0 ± 0.7 |
0.32 |
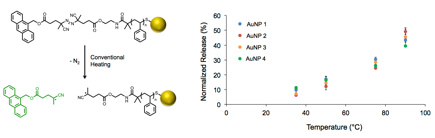
Solutions of decorated gold nanoparticles (0.2 mg/mL) were heated at various temperatures for 1 h, and the increase in fluorescence of each solution was measured immediately thereafter. These measurements were normalized to the complete release of anthracene (90 °C for 18 h) and, the extent of release depends strongly on the oil bath temperature (Figure 3), but is independent of the spacer length due to the homogeneous heating conditions.
Figure 3. Conventional heating calibration curves for AuNPs prepared from PS 1-4
The decorated AuNPs were exposed to microwave irradiation (100 W) for 1 h. The bulk solution temperature (Tbulk) was measured using an external thermocouple, the measured fluorescence value was correlated to the respective calibration curve, and the value for the apparent temperature (Tapp) was obtained. Using Equation 1, the increased surface temperature attributed to microwave irradiation of the gold particles (ΔTlocal) was determined.
ΔTlocal = Tapp - Tbulk (1)
It can be assumed that the azo moiety is present at the average radius of gyration (Rg) of the polymer, allowing us to accurately estimate the effective distance between the azo linker and the AuNP surface using Equation 2:
where l is the length of a single styrenic repeat unit (0.252 nm), Mw is the weight average molecular weight of the polymer, and Mo is the repeat unit molecular weight (104.15 g/mol) (Table 1).
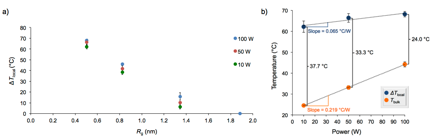
ΔTlocal was plotted as a function of the Rg and the extent of heating shows a strong dependence on the distance between the azo moiety and the nanoparticle surface (Figure 4). In close proximity to the nanoparticle, the temperature increase reaches nearly 70 °C, however, heating effects are not observed 2 nm from the particle surface.
Figure 4. (a) Plot of the change in local surface temperature as a function of distance from the nanoparticle surface and (b) bulk temperature increase is much faster than local temperature increase as a function of power.
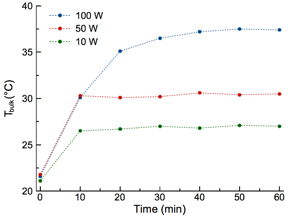
Furthermore, kinetic studies were performed to examine the heating and fluorescence release during the entire irradiation time. The bulk temperature raised rapidly to its maximum temperature, then remained constant (Figure 5).
Figure 5. Plot of bulk temperature as a function of microwave irradiation time.
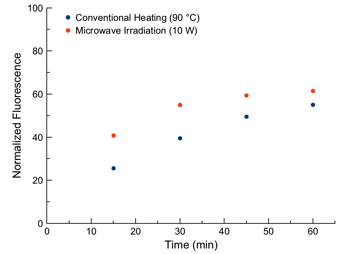
A similar release profile is observed for microwave irradiation and conventional heating (Figure 6). Moreover, the exponential decay indicates that the local temperature remains consistent throughout the irradiation time (as azo dissociation exhibits exponential decay decomposition kinetics).
Figure 6. Kinetic study measuring percent release of anthracene fluorophore
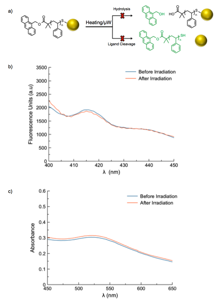
Control reactions in which the fluorescent tag was linked directly to the polymer (no azo linker) verified the mechanism of fluorescence release (Figure 7).
Figure 7. (a) Schematic of control reactions, (b) fluorescence spectra, and (c) UV-Vis spectra before and after irradiation.
The results of this study strongly support the proposed localized superheating of gold nanoparticles under microwave irradiation. The fundamental information gained in this study will promote increased use of AuNPs combined with microwave irradiation to complete chemical transformations selectively at the nanoparticle surface. With the knowledge gained, it may now be possible to tune our originally proposed system for surface selective polymerization.