Reports: ND753971-ND7: Interfacial Free Radical Polymerization of Thin Films
Kevin A. Cavicchi, University of Akron
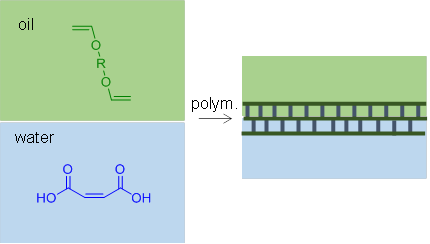
The motivation for this work is the potential use for these materials as barrier layers in thin film composite (TFC) membranes for separations.3 TFC membranes consist of a porous polymer support coated with a thin, selective barrier layer. Current barrier layers are formed by interfacial condensation polymerization, such as between amine and acid chloride containing monomers to produce defect free, polyamide films for desalination membranes. While polyamide barrier layers produce high separation efficiencies they are unstable in the presence of chlorinated water.4 Interfacial free radical polymerization of films would open up a wider range of chemistries for the synthesis films in composite membranes including desalination, gas separation, and pervaporation membranes.
Figure 1. Schematic of interfacial free radical polymerization.
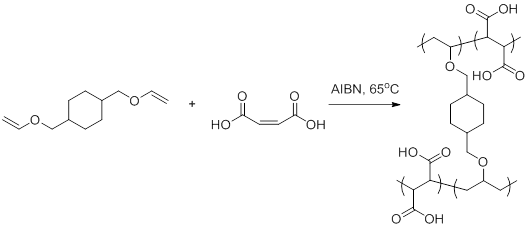
Maleic acid (MA - hydrophilic) and 1,4-cyclohexanedimethanol divinyl ether (CHDVE -hydrophobic) were chosen as the model monomer pair to synthesis alternating copolymers (P(CHDVE-alt-MA)). As CHDVE contains two vinyl ether groups it acts as a crosslinker producing an alternating copolymer network (Figure 2).
Figure 2. Alternating free radical polymerization of MA and CHDVE to produce P(CHDVE-alt-MA).
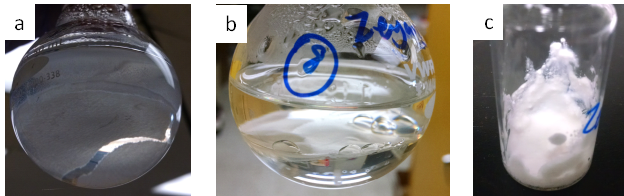
Interfacial polymerization occurred when the monomers were dissolved in a two layer oil/water mixture (1-3M concentration) and heated in the presence of free radical initiator (AIBN) dissolved in the oil phase. As shown in Figure 3 the appearance of the polymer film depends on the choice of solvent. In hexane an opaque, brittle film is formed at the oil/water interface, while in toluene a more transparent and flexible film is formed at the oil/water interface. Other solvent pairs investigated were chloroform/water, and ethyl acetate/water. In chloroform/water a very weak film was formed that could not be handled without breaking. In ethyl acetate/water the initial two phase solution became a miscible one phase solution at elevated temperature. As a control experiment the monomer pair was also polymerized in THF, after rotovapping the solution a white, powder like polymer was obtained (Figure 3c). As the films produced in toluene/water could be removed from the reaction solution intact further experiments were conducted using this reaction system.
Figure 3. Interfacially polymerized films in (a) hexane/water, (b) toluene/water and (c) control sample polymerized in THF. The molar ratio of CHDVE:MA = 1:2 (a, c), 2:3 (b).
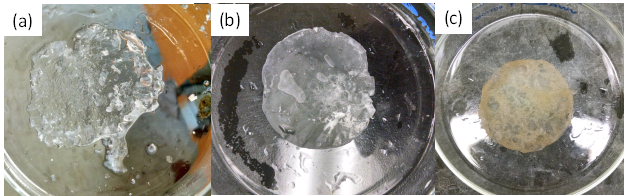
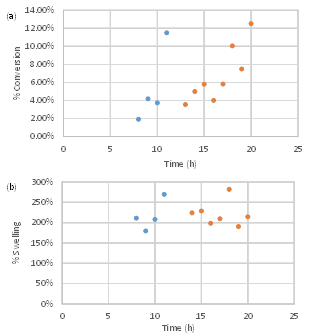
Figure 4 shows films immediately after removing from the toluene/water interface for different polymerization times. In this solvent system the films are swollen with both toluene and water. For example, after drying the film polymerized for nine hours and reswelling, it had a swelling ratio Q (weight swollen/weight dry) of 1.5 in toluene and 1.8 in water. The gravimetric conversion of the polymerization and percent swelling of the as-polymerized film as a function of time are shown in Figure 5a and b, respectively. Here two molar ratios of CHDVE:MA were examined 1:2 (1.2 M CHDVE, 2.3-3 M MA) and 1:1 (1.1 M CHDVE, 1.2 M MA). Monotonic increases in the conversion are observed. The slower rate for the 1:1 CHDVE:MA system may be due to the lower monomer concentration in the MA phase. However, the % swelling of these films was similar indicating that the structure may be similar.
Figure 4. Interfacially polymerized P(CHDVE-alt-MA) in toluene for (a) 6h, (b) 9h and (c) 24h. CHDVE:MA molar ratio = 1:1.9 (a,c) 1:2 (b).
Figure 5. (a) Gravimetric conversion and (b) % swelling of as-polymerized P(CHDVE-alt-MA) in toluene water at CHDVE:MA ratios of 1:2 (blue) and 1:1 (red).
The significance of the work thus far is that has been demonstrated that interfacial free radical polymerization may be used to prepare bulk polymer films at an oil/water interface. Future experiments will investigate first, film properties (i.e. reaction conversion, % swelling) over a wider range of formulation conditions (e.g. CHDVE:MA ratio, monomer concentration, varying AIBN concentration) and monomer chemistries (e.g salt neutralized MA, other divinyl ether monomers). Second, the polymerization of films in polyethersulfone membranes will be investigated to generate composite films.
This ACS-PRF funding has allowed the PI research group to use their expertise in free radical polymerization to branch out into a new direction with potential important applications in membrane separations useful to the petroleum industry. The first year of research of one Master’s student and one PhD student have been supported by this grant.
(1) Braun, D.; Hu, F. Prog Polym Sci 2006, 31, 239-276.
(2) Scott, C.; Wu, D.; Ho, C. C.; Carlos, C. J Am Chem Soc 2005, 127, 4160-4161.
(3) Ismail, A.; Padaki, M.; Hilal, N.; Matsuura, T.; Lau, W. Desalination 2015, 356, 140-148.
(4) Do, V. T.; Tang, C. Y.; Reinhard, M.; Leckie, J. O. Environmental Science & Technology 2012, 46, 852-859.