Reports: DNI754249-DNI7: Bottom-Up Synthesis of Freestanding Two-Dimensional Polymers with Long-Range Order
Lei Fang, PhD, Texas A&M University
Two-dimensional (2D) materials have drawn a lot of attention in scientific research because of their excellent chemical and physical properties. Despite the great amount of efforts have been made, however, this research field remains limited by the challenges on the large-scale production and the challenges on developing new materials with structural versatility. In this project, we design a practical bottom-up synthetic approach to construct free-standing 2D polymers by using thermal dynamic covalent bond interaction or non-covalent bond interaction. Such an approach will allow for large-scale production of organic 2D materials from inexpensive starting materials. Moreover, the bottom-up strategy enables structural variation and allows systematic investigation of the structure-property relationships of 2D compounds.
1. Two-dimensional polymers through dynamic covalent chemistry.
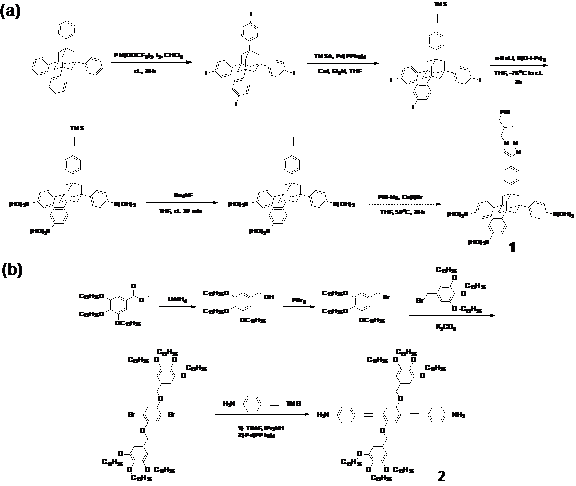
Scheme 1. Synthetic route of two different monomers for 2D polymers.
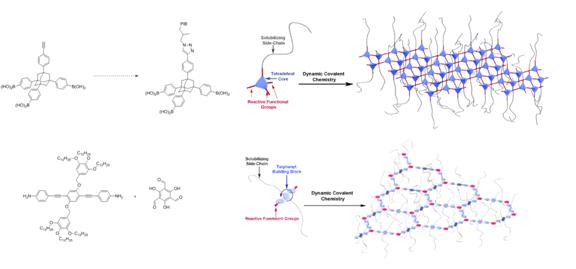
At current stage, this project is mainly focusingon facilesynthetic approaches to the building blocks necessary for the designed 2D polymers. As show in Scheme 1, two monomers with different symmetry (C3v for 1and C2 for 2) were synthesized from commercial available starting materials (1-bromoadamantane for 1 and methyl gallate for 2). Extremely long and flexiblesoluble side chains, such aspolyisobutylene (PIB) and dodecanyl, are introduced by click reaction and SN2 reactions, respectively. At present, compound2has been achieved in four steps while precursor of 1has beenreached in five steps. Using this strategy, these two monomers showed high solubility in common organic solvents, which facilitates the solubility of the expecting 2D polymers. The 2D polymers will be synthesized through dynamic covalent chemistry (DCC) such as boronic ester reaction and amine condensation reaction. We anticipate these two 2D polymers possess solution processability as well.Researchtowards the synthesis and investigation of their physical properties is in progress.
Figure 1. Graphical representation of the construction of two different 2D polymers.
2. Assembly of 2D polymers from 1D small molecules/polymer using non-covalent interaction
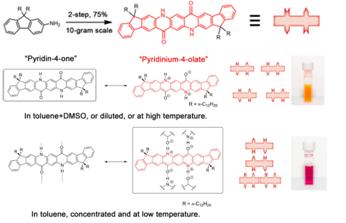
Another viable approach to achieve ordered 2D polymer structure involves the utilization of non-covalent bonds, which possess intrinsic dynamic nature. We have explored the use of hydrogen-bonds (H-bonds) to achieve this goal. As shown in Figure 2, core-extended quinacridone derivatives with intact H-bonds were synthesized by a novel "condensation followed by annulation" strategy. This highly efficient, environmentally benign, and cost-effective route enabled the preparation of these soluble quinacridone-derived materials in large scale. Their H-bonding ability was confirmed by solution-phase 1H NMR and UV−vis spectroscopy experiments. H-bonding promoted 2D networks formed in solid state. Uniform films can be easily cast by spin-coating. Facile synthesis and unique combination of properties of IFQA derivatives render them promising building blocks for the construction of materials for high-performance dyes and optoelectronic devices. Currently, we are working on extending the structure of the H-bonding molecular into 1D polymer so that a dynamic assembly of these chains will lead to a 2D supramolecular polymer, if the side-chains are carefully chosen.
Figure 2. Assembly of 2D polymers from 1D small molecules using H-bonding interaction.
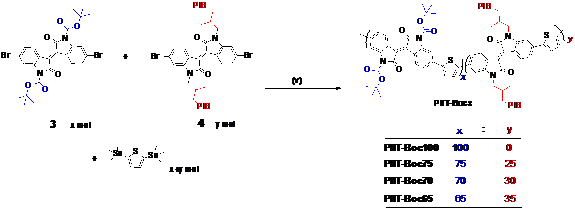
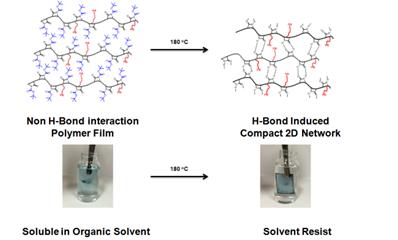
Along the same direction,a series of solution processable conjugated random copolymers with latend H-bondswere synthesized by Stille coupling reactions. Tert-Butyloxycarbonyl (t-Boc) was introduced because it could be easily removed by heating to give H-bonding donor and acceptor in polymer backbone. In addition, Polyisobutylene (PIB) side chain was introduced to give sufficient solubility without compromising electronic performance. By post fabrication thermal treatment, the t-Boc group could be removed efficiently to generate a compact 2D H-bonding network in the solid state, leading to a solvent resistant semiconducting thin film (Figure 3).This unique property makesthese polymers as novel solvent-resistant materials applicable in harsh condition. OFET devicesdemonstratethat the annealed films maintained the charge carrier mobility even after being immersed in different solvents. In addition, the latent H-bonding protected materials by t-Boc also have potential in making very thick film by solution processing and multilayer device.
Scheme 2. Synthesis of the copolymers PIIT- Bocy using Pd-catalyzed Stille coupling reactions, via a random copolymerization approach.
Figure 3. Assembly of polymer networks through H-bonding interaction.