Reports: DNI653103-DNI6: Spectroscopic Characterization of Pt(II) Catalytic C-H Activation Intermediates
Etienne Garand, PhD, University of Wisconsin (Madison)
The proposed research aims to isolate and characterize reaction intermediates in C-H activation catalyzed by Pt(II) complexes. The experiment combines electrospray ionization (ESI), mass spectrometry and infrared vibrational predissociation spectroscopy, allowing for the isolation of the crucial s-CH complexes and other intermediates, and providing detailed structural information on these complexes. The results can shed light on the metal-CH interactions that potentially dictate the catalytic selectivity and rate.
Since the start of the grant, we have finished the construction of our instrument. The apparatus consists of a home-built ESI source capable of soft transfer of intact molecules from solution to the vacuum of mass spectrometer. To controllably generate and isolate the complexes of interest, we installed a gas phase reaction trap after the ESI source. Specifically, a suitable precursor from the ESI source can be trapped in the reaction cell, and via collisions, desired species can form either by fragmentation or by reaction with a gaseous reactant. Figure 1 shows the mass spectral results of the collisional activation and reaction of the platinum ethylenediamine (Pt(en)Cl) complex with benzene. With only buffer gas in the trap, the acetonitrile ligand can be removed via collisions in the reaction trap. This leave a relatively reactive species, which readily binds a water molecule present in the background. When benzene is introduced with the buffer gas into the reaction trap, the desired Pt(en)Cl(C6H6) complex is formed. The results shown in Figure 1 is acquired under room temperature conditions. Similar experiments have also yielded the Pt(en)Cl(CH4) complex, the first step in capturing the s-CH interaction. This reaction trap is currently being fitted with liquid nitrogen cooling and precise temperature regulation capabilities, allowing for optimal formation of complexes with varying binding energies.
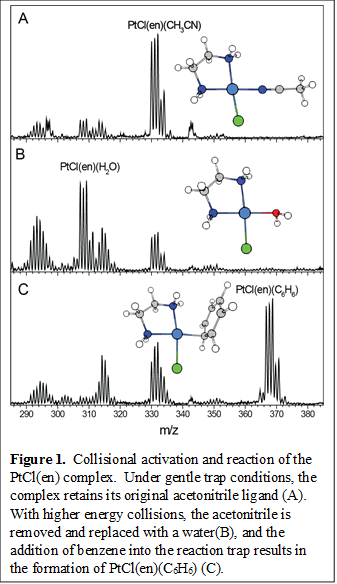
The ions of interest, once formed in the reaction trap, are guided into a cryogenic 3D quadrupole ion trap, typically held at 10 K. There, the ions are collisionally cooled and tagged with weakly bound D2 molecules. Pulsed extraction from the quadrupole trap sends all the ions into a reflectron time-of-flight mass spectrometer, which yields mass spectrum with m/Dm of ~2000. The mass-selected D2-tagged ion packet is intersected with the output of an OPO/OPA infrared laser system, tunable in the 600 - 4500 cm-1 region. When the photon energy is resonant with a molecular vibration, the absorption of a single photon is sufficient to induce the rapid dissociation of the D2 tag. An IR spectrum is obtained by monitoring the intensity of the photofragment ion as a function of the photon energy. We have already used the instrument to study the charge transfer dynamics in metal hydroxide cluster, the structures of peptides, and characterized water oxidation catalysts.
Shown in Figure 2 are the infrared vibrational predissociation spectra of the bare Pt(en)Cl catalyst and the complex with benzene or water. The results illustrate the structural information we can obtain from our experiments. For all three spectra, there are a set of peaks at ~3300 cm-1, typical of N-H stretch. The bare catalyst has a set of four peaks for the four N-H bonds, showing clearly the trans-effect, where having a chloride opposite a NH2 group influences its N-H bonds. The same quartet peaks are observed for the Pt(en)Cl(H2O) spectrum with only minimal changes in their frequencies, indicating that the water ligand does not have a significant trans-effect. Interestingly, the O-H stretches are significantly redshifted compared to those of a free H2O and they are broader than the N-H stretch peaks. It is likely that the negatively charged chloride ligand has a slight interacting with the nearby water ligand, giving rise to these effects. The C-H stretch of the ethylenediamine is visible in all three spectra at 2950-3000 cm-1. The C-H stretch of benzene appears at slightly higher frequencies. We observe the ~3060 cm-1 peak, which is characteristic of benzene, but the ~3160 cm-1 feature is unique to this complex. Additionally, the benzene ligand shows a significant trans-effect, redshifting the frequencies of the NH2 moiety trans to it, such that the N-H stretch region now shows only one pair of peaks.
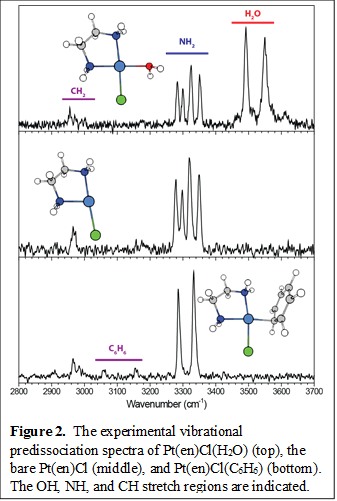
Detailed analysis of these results are currently underway, where careful comparisons of the experimental IR spectra with calculations should reveal the underlying interactions giving rise to these characteristic frequencies. Future work will be aimed at carrying out the same set of experiments for the Pt(en)Cl(CH4) complex in pursuit of the elusive s-CH complex. The Pt-CH interaction should give rise to a rather distinctive C-H stretch with a frequency below those of the CH stretches observed so far. The newly added temperature control capability in the reaction trap will give better control for creating the delicate s-CH complex, and also allow us to add solvent molecules to the complex if additional stabilizations are needed.
The grant has been used to support one graduate student and part of the PI’s summer salary. The graduate student participated in the construction of the instrument and the experiments outlined above. In the process, he has gained invaluable knowledge and experiences in instrumentation and data analysis, and an overall deeper understanding of fundamental molecular properties and interactions. He is currently continuing the experiments while mentoring new graduate students in the lab. The grant was also used for the graduate student’s travel to conferences, where he interacted closely with other scientists in similar fields. This grant has also helped the PI by yielding preliminary data for federally-funded grant proposals.











