Reports: ND653930-ND6: Multifunctional Desulfurization Elastomeric Polymer Nanocomposites
Zhanhu Guo, Lamar University
Suying Wei, Lamar University
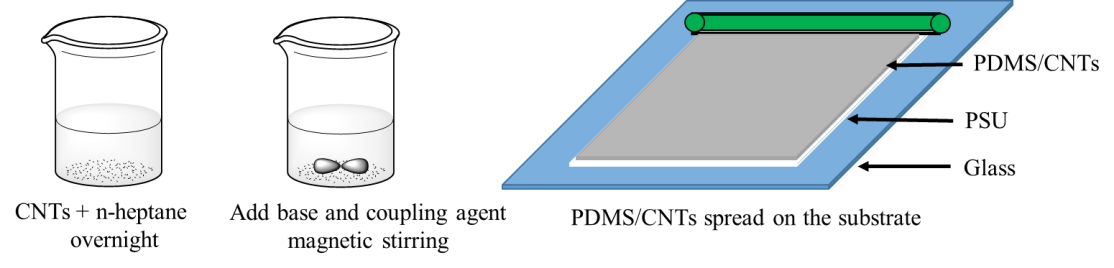
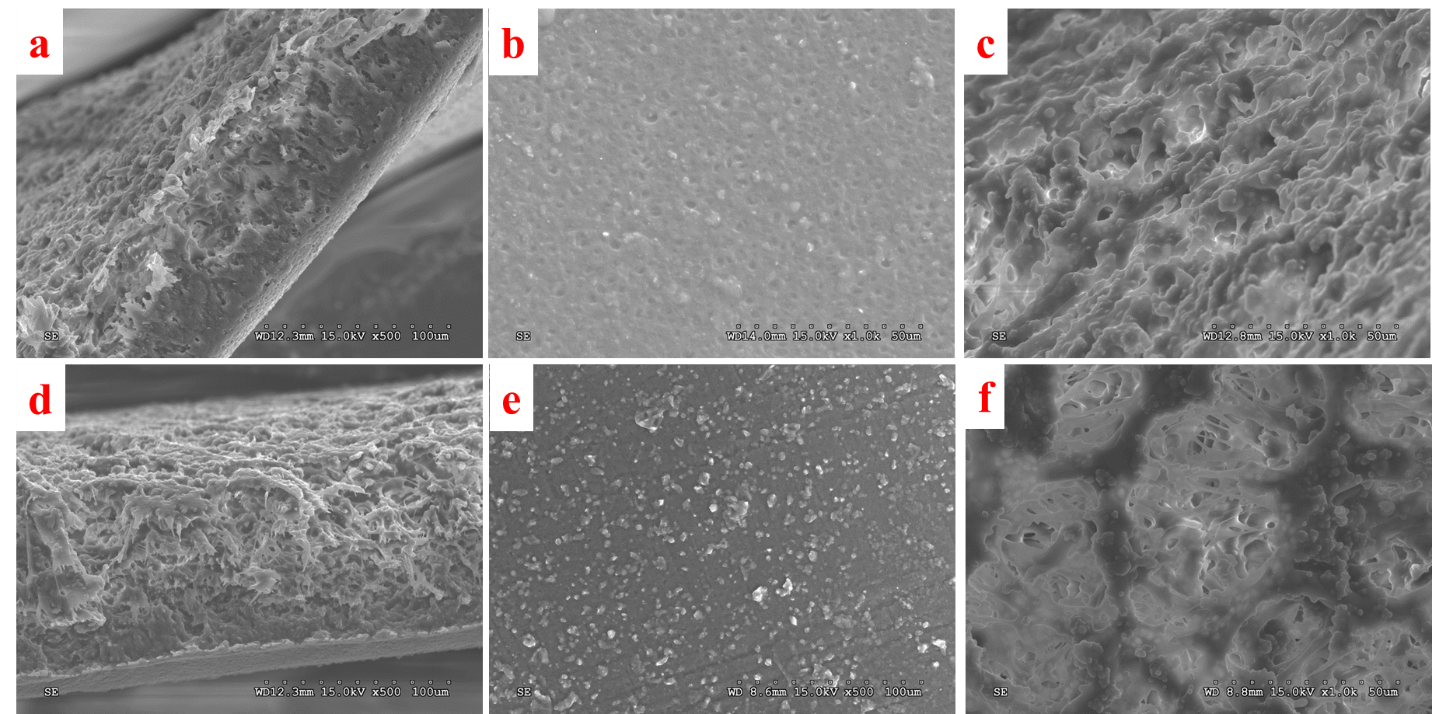
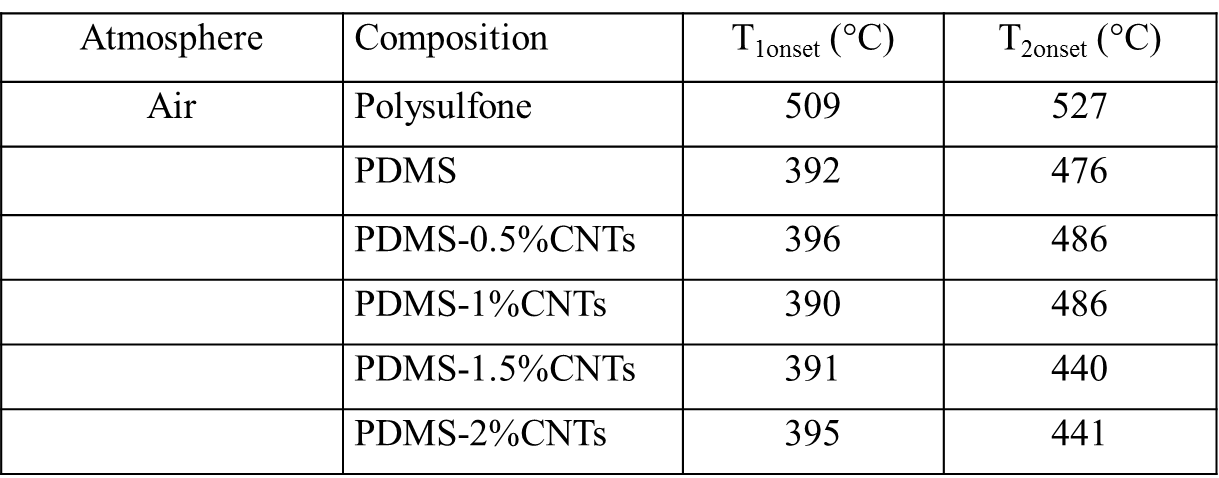
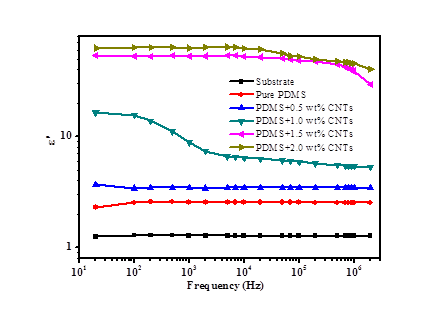
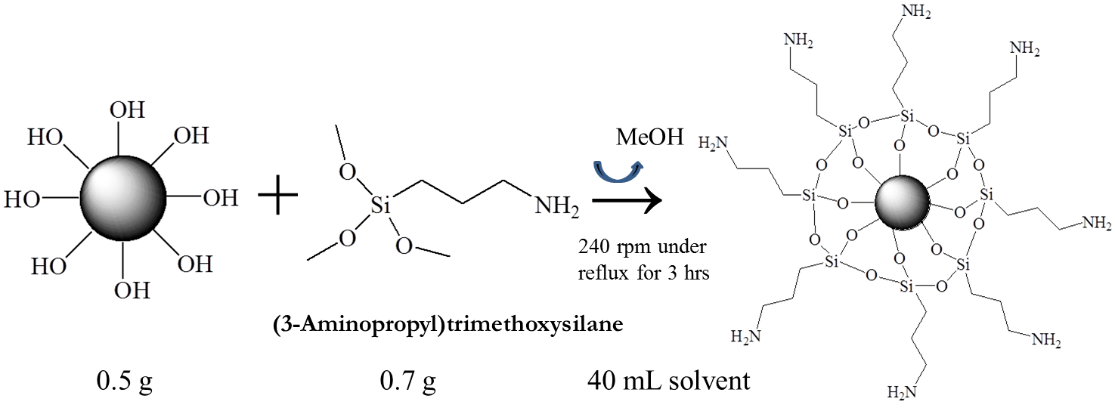

Zhanhu Guo, Lamar University
Suying Wei, Lamar University




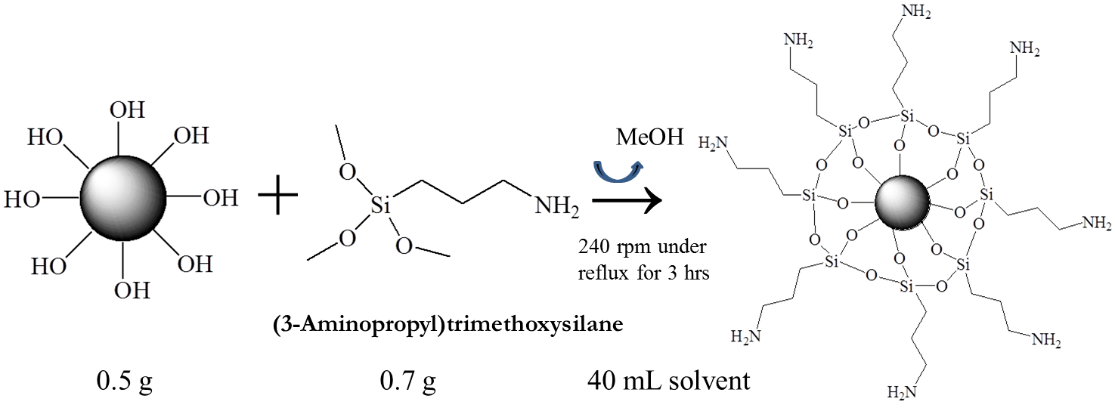

Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society