Reports: UNI353848-UNI3: Development of Homogeneous Ru(II) Olefin Hydroarylation Catalysts Supported by Oxy-Anionic Functionalized Bis(pyrazolyl)alkane Ligands
Brandon Quillian, PhD, Armstrong State University
Brief Synopsis: This grant report under review is focused on the preparation of homogeneous Ru(II) olefin hydroarylation catalysts supported by oxy-anionic functionalized bis(pyrazolyl)alkane ligands. While we have made significant synthetic progress towards a viable catalyst, we have not, as of yet, been able to isolate the targeted compound. Herein, we present our progress and some of the difficulties we have faced and our new methods to circumvent some of these issues.
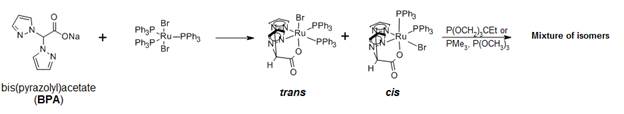
Research progress: We previously showed that the reaction of bis(pyrazolyl)acetate (BPA) with RuBr2(PPh3)3 led to the formation of a mixture of cis/trans isomers of (BPA)RuBr(PPh3)2 (Scheme 1). We surmised that replacing the PPh3 ligands of these compounds with ligands stable to C–H activation, followed by phenylation of the metal, would afford a viable catalyst. However, the substitutions have proved problematic. We have since taken a different approach.
Scheme 1. Preparation of (BPA)RuBr(PPh3)2 and attempts to substitute the PPh3 ligands.
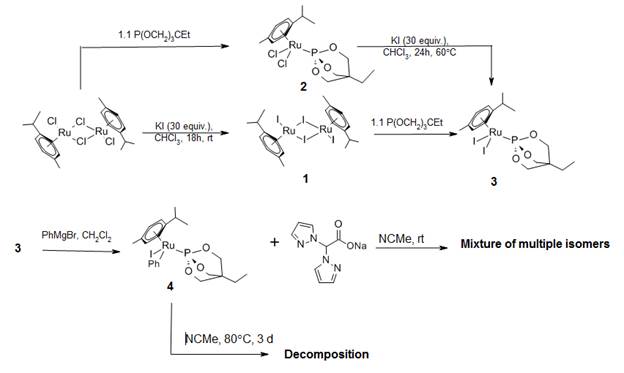
We recently published an article disclosing the displacement of the cymene ligand of [(cymene)RuBr2]2 with tris(pyrazolyl)methane (HC(pz)3; pz = pyrazolyl) and facile phenylation of ruthenium from the Ru–Br bond. We employed this strategy in our ongoing studies with the BPA ligand, but instead from the Ru–I bond. Scheme 2 shows work performed relevant to this strategy and should be referred to for the following discussion. We were able to prepare the integral starting material [(cymene)RuI2]2 (1) by a modified literature approach. Reaction of 1 with P(OCH2)3CEt yields (cymene)RuI2(P(OCH2)3CEt (3), which can also be produced by reaction of (cymene)RuCl2(P(OCH2)3CEt (2) with KI under reflux. Surprisingly, compound 3 reacts with commercially available PhMgBr in CH2Cl2 at room temperature to give (cymene)RuPhI(P(OCH2)3CEt (4) with no signs of decomposition due to solvent reactivity. We attempted to replace the cymene ligand in 4 with BPA, but this resulted in a mixture of isomers and loss of the phenyl ligand. Refluxing 4 in acetonitrile followed by reaction with BPA does not afford the desired product either.
Scheme 2. Reactions of [(cymene)RuI2]2 to prepare a viable catalyst
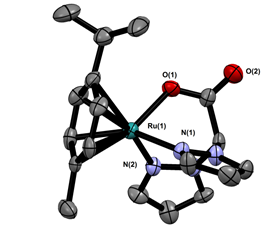
To circumvent the formation of multiple isomers, we are now using a single ancillary ligand, acetonitrile, due to its lability with soft transition metals. The small cone angle of acetonitrile also provided an opportunity to examine bulkier ligands such as bis(3,5-dimethylpyrazolyl)acetate (3,5MeBPA). Scheme 3 portrays the work performed thus far. Reaction of BPA or 3,5MeBPA with 1 in the presence of potassium carbonate in acetonitrile at room temperature yields (cymene)Ru(BPA/ 3,5MeBPA) compounds, 5 and 6, respectively. We have fully characterized 5 including its X-ray structure (Figure 1).
Figure 1. Single X-ray structure of (cymene)Ru(BPA), 5.
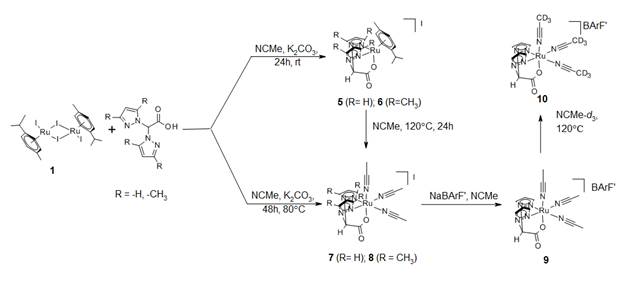
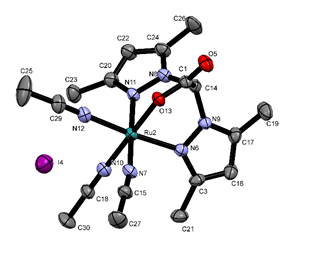
Increasing the reaction temperature to 120°C led to the formation of tris(acetonitrile) complexes 7 and 8. Compounds 7 and 8 can also be prepared by reaction of 5 or 6 with acetonitrile at 120°C, suggesting 5 and 6 are intermediates in the formation of 7 and 8, respectively, starting from 1 and BPA. The single crystal X-ray structure of 8 is shown in Figure 2. Compounds 7 and 8 have poor solubility in benzene (the solvent used in future olefin hydroarylation studies), which can be improved by exchange of the iodide anion with tetrakis(3,5-bis(trifluoromethyl)phenyl)borate (Na[(3,5-(CF3)2C6H3)4B]) (9). The thermal stability and lability of the acetonitrile ligands of complex 9 was gauged by ligand exchange reactions with acetonitrile-d3 at 120˚C, showing loss of the acetonitrile 1H NMR signals and no signs of decomposition. These observations suggest this ligand type is stable and practical for catalytic studies. We have begun to perform C–H activation studies with 9 but have not obtained conclusive evidence for C–H activation of benzene.
Schem 3. Reactions of [(cymene)RuI2]2 with BPA in acetonitrile
Figure 2. Single crystal X-ray structure of [(BPA)Ru(NCMe3)]I, 8.
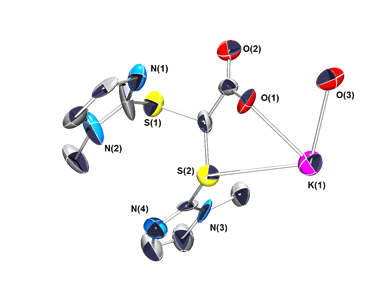
Scheme 4 should be referred to for the following discussion. We have attempted to prepare bis(methimazolyl)acetate (11) using conditions employed for the preparation of BPA; however, these reaction systems tended to decarboxylate to form the known bis(methimazolyl)methane (12). We have incorporated conditions to limit the decarboxylation event; however, the more kinetically accessible S−C bonds are formed to create 13. We were able to determine the single crystal X-ray structure the potassium salt of 13 (Figure 3). To our knowledge, a ligand of this archetype is unknown. Examination of the coordination properties of 13 with either H[AuCl4] or RuBr2(PPh3)3 led to S–C bond scission and formation of interesting compounds (14 and 15) with monodentate sulfur coordination to the metals (Scheme 5). An article highlighting the structural features of the gold complex (14) was accepted into Acta Crystallographica Section C.
Scheme 4. Attempts to prepare bis(methimazolyl)acetate, 11.
Figure 3. Single crystal X-ray structure of potassium S,S-bis(methimazolyl)acetate, 13.
Scheme 5. Reaction of 13 with transition metals
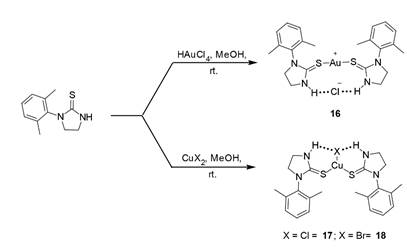
We attempted the preparation of larger N-substituted derivatives of 13 but were unable to isolate such species. However, bis(1-(2,6-xylyl)-2-imidazolidinethione was prepared and reacted with H[AuCl4], Cu(II)Br2, Cu(II)Cl2 to yield some of the most sterically encumbered gold and copper bis(heterocyclic thiourea) transition metal complexes (16-18) currently known (Scheme 6). The results of these studies have been accepted for publication in Structural Chemistry and Crystallography Communications.
Scheme 6. Reaction of Bis(1-(2,6-xylyl)-2-imidazolidinethione with gold and copper salts.
Faculty and Student Impact: In this era of limited funding opportunities in fundamental research, the generous contribution of the ACS-PRF grant has release a tremendous burden off of the departmental coffers, which supports numerous faculty research projects. I have been able to devote more time to this research project over the summer semester and was able to employ deserving undergraduate students, who have obtained extensive training with synthetic design, scientific methods, instrument operation, spectral interpretation and handling of air- and moisture sensitive compounds. A total of seven research presentations were disseminated and three research articles were submitted over the last year. These publication has granted name exposure to the scientific community, leading to numerous collaborations and review request. We hope to finish the preparation of one catalyst and begin our catalytic studies during the final year of this grant.