Reports: ND754253-ND7: Understanding the Effect of Graphene Oxide's C:O Ratio on Particle Polymer Intermolecular Forces and Resulting Macroscopic Properties
David E. Kranbuehl, PhD, The College of William and Mary
Our research has focused on understanding at the molecular level the relationship of the surface chemistry of graphene oxide (GO) to its ability to be dispersed as single isolated nano- sheets and to the resulting change in intermolecular forces between GO and the polymer. The objective is a fundamental understanding at the molecular level of how modifying the GO surface chemistry can achieve large improvements in the desired properties at very low loadings, down to one part per ten thousand. Initially the focus in the proposal was on characterizing the increase of the GO C:O ratio after exposure to heat and UV light and the resulting shift from hydrophilic to hydrophobic as well as development of attractive intermolecular forces between GO and the polymer.
Realizing the limitations of a focus on the C:O ratio, the research shifted to a broader approach: functionalizing the GO surface by reacting The GO hydroxyl and epoxy groups with molecules compatible with the polymer. The past six months we focused on functionalizing the GO surface to be compatible with polyamides and polyimides and on understanding the relationship of the functional group chemistry to changes in the GO-polymer properties. Of particular interest are reduction of hydrolytic degradation in polyamides, decreasing water vapor transmission in polyimides, and improvements in stiffness. We propose GO significantly improves these macroscopic properties because at the molecular level the GO nanosheet inhibits polymer backbone mobility. We are using changes in the rates of nucleation and crystal growth to verify The GO effect on polymer chain mobility and thereby hydrolysis and water vapor diffusion.
We have completed work on exploring the effect of functionalized GO versus as- produced GO on the reduction in water vapor transmission in a thermoset polyimide. In the coming year, using a thermoplastic polyimide, we will work on comparing three variations in The GO surface chemistry and resulting variation in intermolecular GO-polymer forces. Using a low temperature polymerization, we will a) optimize C:O ratio of unfunctionalized GO, b) functionalize the GO surface with the amine monomer and c) allow GO to react with polyimide monomers during polymerization and become chemically bound to the polymer chain. Based on our year 1 work described below, it is clear that large changes do occur depending on the functionalization and incorporation of GO into the polymer.
Very recently we have succeeded using atomic force microscopy (AFM) to measure the intermolecular force in between individual GO sheets and the polymer. This is based on our previous work using AFM techniques to measure the relative adhesive strength between GO and a range of different polymers through peeling tests. After many attempts to develop a
method to coat an AFM probe with GO and potential functionalizing molecules, the last few weeks have shown extremely encouraging results. We now have preliminary data suggesting that we can directly measure the attractive and adhesive interactions between different polymers and AFM probes with different functionalizations: nacent AFM probes, modified probes chemically coated, and GO-coated probes.
This suggests once fully developed, we will have a novel method to directly measure the intermolecular GO-polymer forces. As a result we will be able to understand at the molecular level how to design the GO surface chemistry to optimize intermolecular forces and thereby enhance macroscopic properties. This would sharply contrast with our current, educated Edisonian approach to functionalizing GO surfaces.
Five undergraduates during the academic year and summer worked with four graduate students on this project.
Abstracts from two presentations at the Boston MRS meeting and two papers in preparation:
The Effect of Functionalized versus Unmodified Graphene Oxide on Polyimide Nanocomposite Properties
Abstract
Graphene oxide (GO) nanoparticles produced by Tours method and graphene oxide functionalized by 4-4 oxydianiline (ODA) are incorporated at 0.01 to 0.10 weight percent into a polyimide (one part per ten thousand to one part per thousand by weight) made from 3,3- benzophenonetetracarboxylic dianahydride (BTDA) and 4-4 oxydianiline (ODA). The performance properties of these GO polyimide systems at these extremely low GO concentrations are compared. The functionalized GO polyimides properties are comparable to previous results at concentrations 10 times higher than our loadings. The ODA-GO functionalized films displayed significantly greater improvement in performance properties.
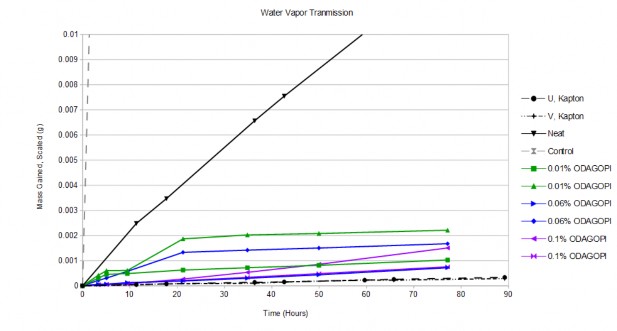
Water vapor transmission measurements to quantify gas barrier properties, water gain analysis measurements to quantify solvent resistance, thermogravimetric analysis to determine thermal stability and mechanical tensile test were made. The 0.01% by weight
ODA-GO polyimide film demonstrated the largest 8 fold decrease in water vapor transmission. The 0.10% by weight ODA-GO film displayed the maximum increase of 82% in the modulus. The 0.06% functionalized GO-ODA PI film showed the maximum 27% decrease in water absorption. All GO-ODA PI films showed little change in polyimides high level of thermal stability to over 400C.
These large increase in desired properties result from functionalizing GO which increases GO-polymer molecular interaction plus the GO highly asymmetric nano planar sheet structure, the hydrophobic nature of thermally reducing GO during polymerization and the GO high modulus.
Figure 1: Water Vapor Transmission results for functionalized GO loaded into Polyimide.
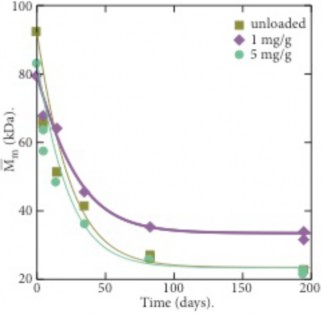
Graphene oxide (GO) is incorporated into polyamide-11 (PA11) via in-situ polymerization to make a composite that has significantly reduced hydrolytic degradation during accelerated aging. At a loading of 0.1 percent by weight, the addition of GO reduced the hydrolysis rate constant 38% at 120C and 65% at 100C resulting in a significantly higher equilibrium molecular weight of 33 and 34 kDa at 100 and 120 C compared to 23 and 24 kDa at 100 and 120 C for unloaded PA11. The decrease in the rate of degradation and resulting 50% increase in PA11 equilibrium molecular weight is attributed to the highly asymmetric, planar nanosheets of GO that we suggest inhibits molecular mobility of water and the polymer chain. Similar effects on polymer mobility were found by measuring the polymer crystallization properties.
Figure 2: Molecular weight change at 100C in water for PA11 and GO loaded PA11.