Reports: ND753739-ND7: Nanostructured and Nanoporous Composites from Nanoparticle Jamming during Polymerization Induced Phase Separation
Bryan D. Vogt, University of Akron
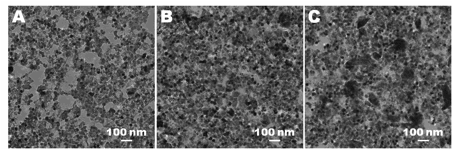 | |
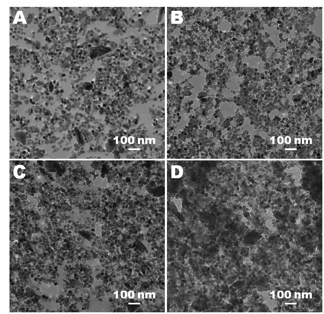
Figure 2. To better understand how the system impacts the structure
that develops, we have performed similar experiments using ethyl acrylate (EA)
as the monomer, poly(methyl methacrylate) (PMMA) as
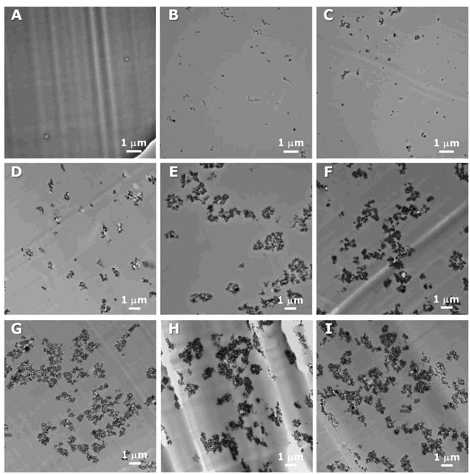
the polymer, and alumina nanoparticles. Figure 3 illustrates the range of
structures that develop upon polymerization. Although it difficult to
distinguish between PEA and PMMA domains in the blend (as shown in Figure 3A),
the TEM micrographs of the nanocomposites show that
the size of clusters constituted of Al2O3 nanoparticles
dispersed in the PEA/PMMA blends increases as a function of Al2O3
nanoparticle contents. Strong interaction between particles concurrent with
polymerization appears to lead to some orientation of the structure at high
nanoparticle loadings. The agglomeration of Al2O3
nanoparticles is encouraged by the hydrogen bond formed between the surface
hydroxyl groups of the nanoparticles. These surface hydroxyl groups increase
the tendency to create hydrogen bonds between nanoparticles and directly result
in the formation of aggregates. At less than~5 wt% of
Al2O3 (Figure 3B, C, and D) isolated domains appear to be
present in the structure, whereas the aggregated Al2O3
nanoparticle domains appear to be interconnected at greater than ~5 wt% of Al2O3 (Figure 3E, F, G, H, and
I).
Figure 3.
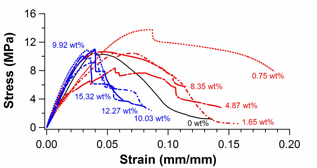
Figure 4.