Reports: ND1053579-ND10: A Rheological Study of Interfacial Bacterial Films: Understanding the Mechanics of Two-Dimensional, Active Materials with Applications to Enhanced Oil Recovery
Robert L. Leheny, Johns Hopkins University
Microbial enhanced oil recovery is an emerging strategy employing
bacteria and their metabolic by-products to increase petroleum yields from porous
reservoirs. We monitored the dynamical behaviour of FBI by
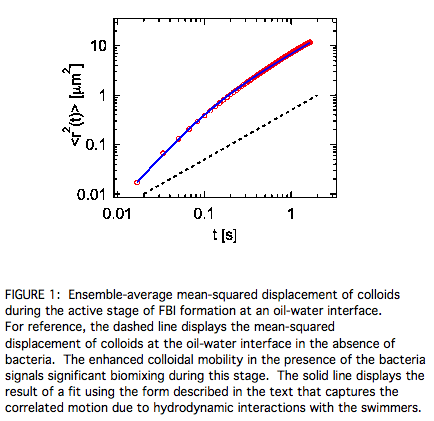
tracking colloids (0.5 micrometer spheres) pinned to the interface between hexadecane and the bacteria solution. Figure 1 shows the
mean-squared displacement <r2(t)> of the colloids during the active
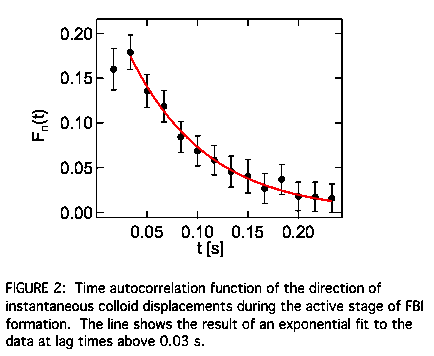
stage of FBI formation. To quantify more directly the tracer velocity correlations,
we identified the instantaneous direction of motion for each particle examined
its time autocorrelation function Fn(t). While the colloid dynamics at large lag times with
diffusivity D suggest that the suspension of swimming bacteria acts like a
thermal bath, as seen previously the statistical properties of the colloidal
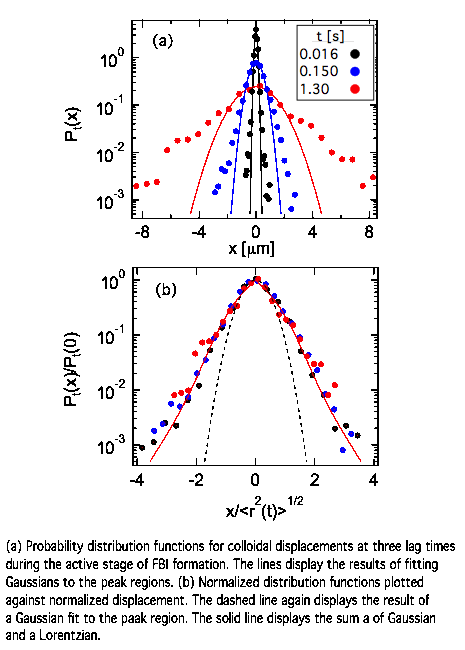
displacements differ from a system in thermal equilibrium. For instance, Fig. 3(a)
shows the probability distribution function (PDF) for displacements at fixed
lag time Pt(x), where x is the
displacement along one direction, at three lag times spanning the superdiffusive and diffusive behaviors.
While the PDFs of particles in thermal equilibrium are Gaussian, the PDFs in
the active FBI show large non-Gaussian tails at large |x| signalling enhanced probability
of large displacements. Qualitatively similar PDFs have been observed among
tracers in other microbial suspensions. To compare the PDFs at different lag
times, we plot in Fig. 3(b) the normalized PDFs with displacement normalized by
the root mean-squared displacement. Remarkably, the PDFs collapse onto a single
lineshape, indicating the distribution maintains a
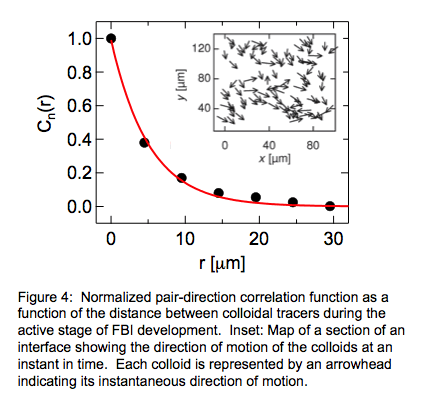
self-similar form with increasing lag time. Another feature of the colloidal motion was their spatial
correlations. As an illustration, the inset of Fig. 4 depicts direction of
motion of colloids at one instant. Alignment between the direction of nearby
colloids is apparent. This coordinated motion is quantified in Fig. 4, which
shows the normalized pair direction–direction correlation function, Cn(r). The spatial correlations decay
exponentially with separation, as depicted by the line in Fig. 4. Together, Fn(t) and Cn(r)
give a quantitative picture of the spatiotemporal correlations in tracer motion
that characterize the dynamical behaviour of the active FBI. Daniel Allan, a Physics PhD student at Johns Hopkins who
led the work, is currently a postdoctoral fellow at Brookhaven National
Laboratory.