Reports: DNI552769-DNI5: Electric Field-Induced Reactions in Fuel Cells From First Principles
Jean-Sabin McEwen, PhD, Washington State University
Methane (CH4), a major constituent of natural gas, can be used to generate syngas (CO + H2) via Ni-based catalytic methane steam reforming (MSR) process. Syngas has many useful applications, such as the Fischer-Tropsch process, in fuel cells, and for ammonia synthesis, so the significance of the MSR reaction is self-evident. The industrial process has two major disadvantages: (i) the formation of coke can rapidly deactivate Ni catalysts; (ii) the highly endothermic MSR reaction is energy inefficient and requires reactor materials with high thermal stability [1]. One way to address these issues is by modifying the selectivity of the catalyst itself. This can be achieved by applying a large electric field to the catalyst. The field rearranges the potential energy states of molecular orbitals, alters adsorbate-surface interactions, and directly changes the overall electrocatalytic activity and selectivity of the catalyst [2].
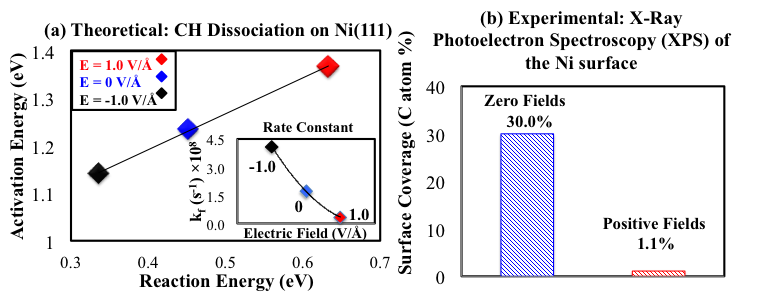
Fig.1. (a) Brønsted-Evans-Polanyi (BEP) correlations for C-H bond breaking, and (inset) rate constants (kf) at 1073 K of CH dissociation; (b) Surface coverage of carbon species estimated from XPS of post-reaction Ni surfaces.
Over the past two years, our research has focused on how an external electric field influences the MSR process at an atomic level by using density functional theory (DFT) calculations. The first study looked at how an external electric field can influence carbon deposits over Ni catalytic surfaces (coking). As shown in Fig. 1(a), our DFT results show that a high positive electric field noticeably decreases the rate constant (at 1073 K) of CH dissociation to form pure carbon atoms on the surface. Accordingly, a positive electric field should impede the formation of coke on Ni surfaces and improve the efficiency of industrial operations. Recent experimental work using MSR appears to validate our theoretical findings [3, 4]. XPS data from our experimental collaborators (Fig. 1(b)) shows a marked decrease in surface carbon coverage with the application of a positive electric field. This information supports designing new electrochemical systems for enhancing Ni-based catalytic MSR processes.
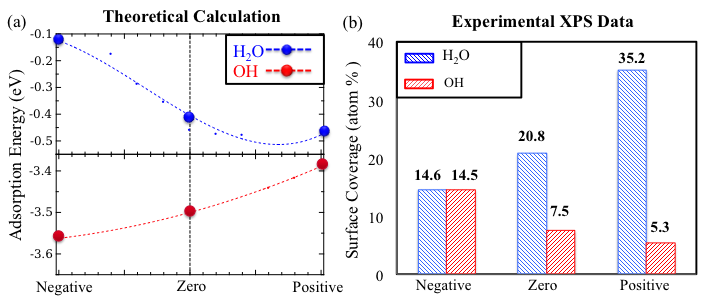
Fig. 2. (a) The adsorption energies of H2O and OH molecules on Ni(111) as a function of an external electric field, (b) Surface coverage of H2O and OH species estimated from XPS of post-reaction Ni surfaces.
To prevent coke formation over Ni catalytic surfaces, industrial operators generally increase the H2O:CH4 ratio to 3:1. Significant energy input is required to vaporize the excess H2O, which decreases the overall efficiency of catalytic MSR processes. The unique permanent dipole moment of a H2O molecule also suggests that H2O may play an important role in electrocatalysis. Thus, the second investigation concerns how electric fields affect the interactions between water molecules and Ni surfaces. XPS data demonstrates that H2O surface coverage increases and OH surface coverage decreases as the applied electric field changes from negative to positive values. This experimental result correlates well with our calculations: Increasing the electric field from negative to positive values, the adsorption energy of H2O over Ni surfaces is monotonically strengthened and the adsorption energy of OH is monotonically weakened (Fig. 2(a)) [5]. Therefore, we can conclude that a positive electric field strengthens H2O adsorption but suppresses H2O dehydrogenation.
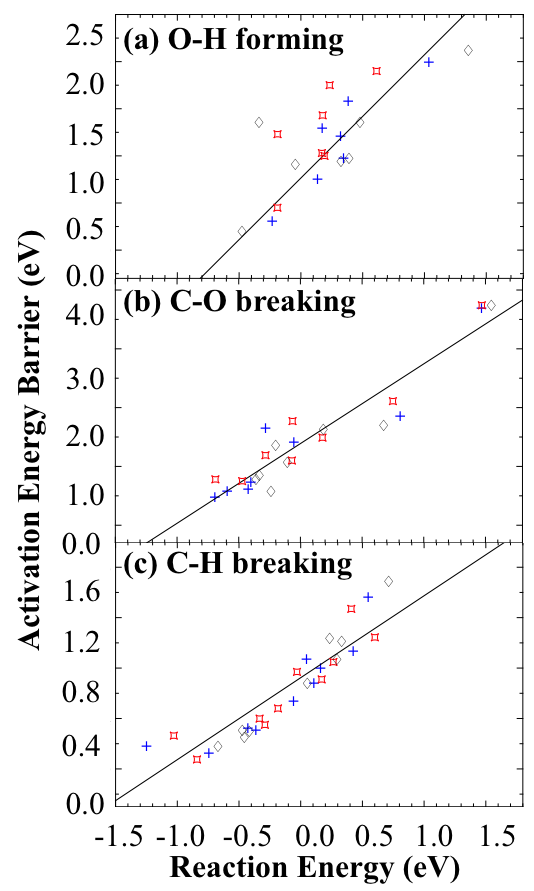
Fig. 3. The BEP correlations for C-H breaking, C-O breaking, and O-H forming reactions during MSR processes with different electric fields. Here, the red, black and blue dots present the energy with positive, negative and no fields.
Another way to increase the efficiency of the MSR reaction is by lowering the reaction temperature. To that end, we have begun to study how fields affect the thermodynamic and kinetic properties of the overall MSR reaction [6]. During the MSR processes, our results exhibit a Brønsted-Evans-Polanyi (BEP) relationship with different electric fields (Fig. 3), which claims that the activation energy for elementary reactions scale linearly with the reaction energy. Such linear correlations indicate that the kinetic properties of any catalytic reaction can be predicted by its thermodynamic properties with different field values. [7]
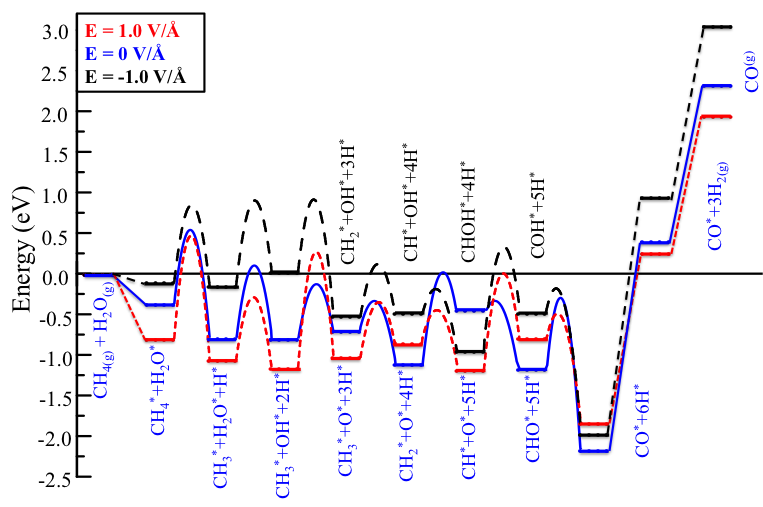
Additionally, an external electric field can change the most underlying mechanism of the MSR reaction: After the application of an electric field, we find CHOH and COH intermediates instead of CHO as seen with in the absence of a field (Fig. 4). The intermediates COH/CHO are also found from the XPS data of the post-reaction Ni surfaces from our corresponding experimental work. The results also show that a positive electric field can stabilize the adsorption of the reactants over a Ni surface and lower the energy requirements for desorbing the CO and H2 products [6, 7].
Fig. 4. The most favorable reaction pathways with different electric fields.
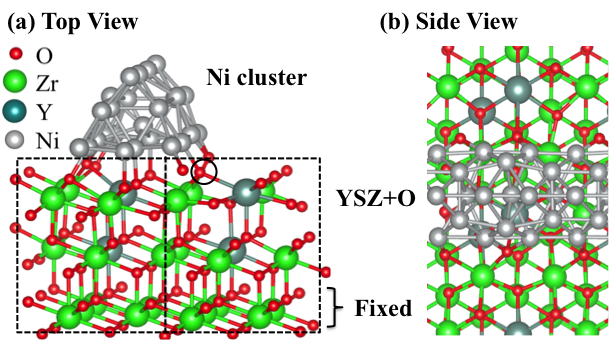
Fig. 5. Model of oxygen enriched yttrium-stabilized zirconia (Ni/YSZ+O) cermet.
MSR processes in Solid Oxide Fuel Cell (SOFC) systems typically use nickel over yttrium stabilized zirconia (Ni/YSZ) as an anode material. To better understand the electronic properties of a Ni/YSZ catalyst, we began by establishing a Ni/YSZ+O model and investigating its interface oxygen vacancy formation (Fig. 5). The results show that the oxygen vacancy formation energy largely decreased from 0.0 eV to -0.6 eV as we changed the field from -1 V/Å to 1 V/Å. Such strong field effects increase our confidence that future work with the MSR mechanism at the interface of the Ni/YSZ catalysts should have highly significant electric field effects compared to those on a pure Ni surface. This ongoing work is important as it marks the first time that such complex and realistic electrocatalytic modeling has been undertaken.
References
[1] S.C. Singhal, Solid State Ionics, 135 (2000) 305-313.
[2] H.J. Kreuzer, R.L.C. Wang, Philos. Mag. B, 69 (1994) 945-955.
[3] F. Che, R. Zhang, A.J. Hensley, S. Ha, J.-S. McEwen, Phys. Chem. Chem. Phys., 16 (2014) 2399-2410.
[4] F. Che, A. Hensley, S. Ha, J.-S. McEwen, Catal. Technol., (2014) 4020-4035.
[5] F. Che, J. Gray, S. Ha, J.-S. McEwen, Accepted by J. Catal., (2015).
[6] F. Che, J. Gray, S. Ha, J.-S. McEwen, In preparation, (2015).
[7] F. Che, J. Gray, S. Ha, J.-S. McEwen, In preparation, (2015).