Reports: UR1053498-UR10: Bottom Up Development of a Gas Hydrate Inhibitor Coating
Paul W. Baures, PhD, Keene State College
ACS PRF Year 2 Report
This project is based on the hypothesis that an inexpensive, durable, and non-toxic polyol-based coating could be developed to inhibit gas hydrate formation in pipelines, building from the knowledge that polyvinyl alcohol and natural antifreeze proteins have both been reported to inhibit hydrate formation.
The specific aims of the project are as follows:
Specific Aim 1. Polyol Synthesis and Characterization
Specific Aim 2. Modification and Characterization of Glass Substrates
Specific Aim 3. Investigating Antifreeze Behavior of Modified Substrates
Review of Year One Effort
In the first year, representative polyols were synthesized, characterized, and used to modify glass substrates. A crude antifreeze protein solution was prepared from mealworms and also covalently attached to a glass substrate. The contact angles of water on these substrates provided evidence of surface modification, but we were unable to show a delay in frosting between positive controls and test samples using a home built observation chamber.
Current Results
During the academic year and this summer the group began efforts to observe the effects of the coatings on tetrahydrofuran (THF) hydrate formation. In this experimental design, the students covalently attached the polyols, antifreeze protein, and linker molecules as negative controls to the inside walls of test tubes through an aminosilane linker, with a goal of showing differences on the growth of THF hydrate in these different test tubes.
A majority of the effort this summer was spent trying to replicate the observed percentage of THF hydrate formation in a solution held just above the freezing point of water. When crystallization occurs in this experiment, an exotherm is observed from an immersed temperature probe. The peer-reviewed literature reports the data as a fraction of experiments that have crystallized with respect to time, and followed each solution out to 24 hours. A large percentage (>90%) of the THF-water solutions are reported to freeze in the first two hours, while addition of polyvinylpyrrolidine (PVP) at 0.25 mM slows the rate of crystallization significantly (~ 50% reduction in the first two hours).
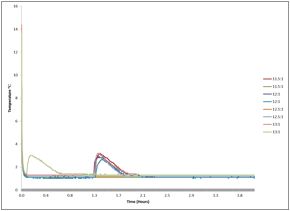
The students first modified a test tube rack to immerse samples of THF-water in a chiller and to include a Vernier temperature probe in each tube. The ratio of THF-water, the temperature of the bath, and the starting temperature of the solutions were all examined as potential factors in the rate of the crystallization. The molar ratio of THF-water in the crystalline state is 1:17, though the literature reports using a slightly more concentrated THF-water ratio, 1:15, in order to favor hydrate formation at 0 °C. We tested this ratio as well as more concentrated THF solutions to see if there was a more favorable nucleation condition. No differences were apparent, indicating other parameters were more important that the ratio alone (Figure 1).
Figure 1. Exotherms associated with hydrate crystallization in solutions containing different ratios of THF-water. The ratio did not appear to be significant to the nucleation event observed in this experiment.
A ratio of 1:12.5 THF-water in a chiller bath of 1 °C was chosen for continued investigation, and students began a series of experiments trying to replicate the observed percentage of hydrate formation reported in the literature. Through multiple experiments, our samples yielded a smaller percentage of the total number of samples that were crystallizing (19% versus 90% as reported). Addition of 0.25 mM PVP decreased nucleation events by an approximate 50% relative reduction in the first two hours in a previous report, while in our case we observed only a 25% relative reduction in nucleation.
We are continuing to refine the execution and design of this experiment this fall. The ability to observe the crystallization of the THF hydrate is a bottleneck in the project, as we need a reliable observation method in order to examine the polyol coatings and positive controls. A standard operating procedure (SOP) will be developed so that multiple students can run their own experiments and still contribute to the overall data of the group. The SOP was begun in the late summer research, but will need refinement as we tweak experimental procedures and methods.
Among the first experiments to be conducted is to return to the 1:15 THF-water ratio reported in the literature, and to move toward 0°C for the chiller. Although the 1:12.5 ratio was chosen to further ensure we would not be observing water freezing alone, it is possible that the added THF is inhibiting crystallization. We will instead include water alone as a control to be sure it is not crystallizing routinely at the same percentage. We will also move closer to 0 °C with the chiller, until our experiments begin giving greater nucleation events in the first two hours, without similar observations for the water alone.
Impact
The support from this grant has provided three researchers with valuable experience in a laboratory environment this summer. A fourth student was supported part-time during this past academic year. Two of the students worked close to full-time on the project during the summer, while one student was part-time. Each student expressed how much the summer research experience meant to them, and the two full-time students presented their research results at the NH-INBRE conference in early August. One has already expressed an interest in pursuing research after their undergraduate degree.
The support for this project has helped build research capacity at KSC, and is changing the culture to where more students are involved in doing research during the academic year and summer. The students are recognizing the value of research to their intellectual progress, and that is helping me as a teacher obtain deeper learning outcomes and as a research progress my own projects. A peer-reviewed publication is going to have to follow refinement of our observation method, though students did present last years effort at the American Chemical Society National Meeting in Denver, CO, in March of 2015. Our current efforts will be presented at local or regional meetings in the coming year, although a specific venue is yet to be decided.