Reports: UR753970-UR7: Surfactant-Induced Multiscale Assembly of Aqueous Conjugated Polymers
Shanju Zhang, PhD, California Polytechnic State University, San Luis Obispo
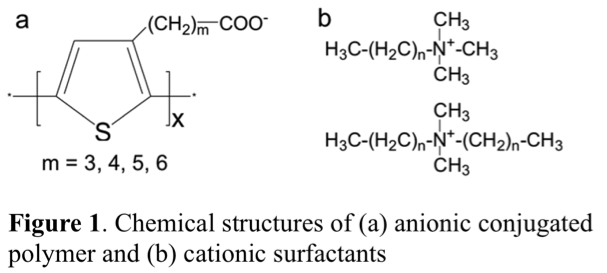
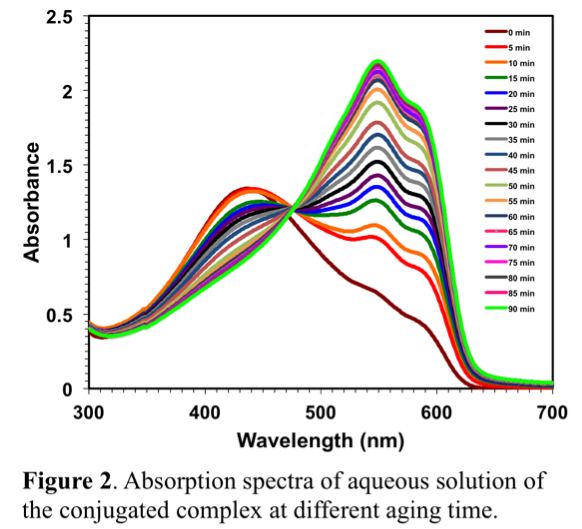
Shanju Zhang, PhD, California Polytechnic State University, San Luis Obispo


Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society