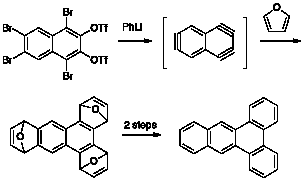
Reports: ND1054328-ND10: Novel Interrupted Polyacenes
Gordon W. Gribble, Dartmouth College
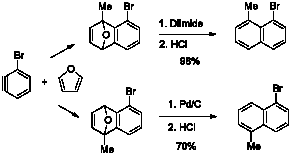
In an offshoot of the main project we performed the Diels–Alder reaction between 2-methylfuran and 3-bromobenzyne, which was generated under mild conditions from 1,3-dibromobenzene and lithium diisopropylamide, to give a mixture of regioisomeric 1,4-dihydro-1,4-epoxynaphthalenes. A subsequent two-step deoxygenation afforded the corresponding 1-bromo-8-methylnaphthalene and 1-bromo-5-methylnaphthalene in high yields. This process illustrates the preparation of two isomeric naphthalene starting materials in one step, since their separation is facile.
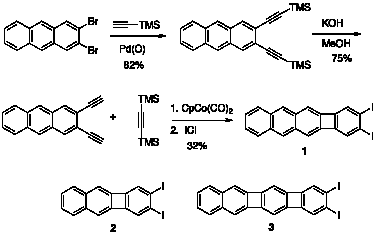
Our main project is on track. We have synthesized several of our proposed target aromatic hydrocarbons and biphenylenes using the methods proposed, including 2,3-dihaloanthracenes and the biphenylene 1 shown here. In similar fashion we have synthesized the new diiodobiphenylenes 2 and 3. We are currently elaborating these key functionalized biphenylenes to the proposed polyacenes.