Reports: ND952789-ND9: Realizing the Selectivity of Exothermic Partial Oxidation Reactions
Anthony G. Dixon, Worcester Polytechnic Institute
1-Introduction
Multitubular fixed bed reactors are
widely used in the chemical industry, especially for partial oxidation
reactions. Due to the fast and highly exothermic nature of these reactions
strong species concentration and temperature gradients exist in the reactor,
leading to loss of selectivity. A greenhouse gas, carbon dioxide, is produced
as a by-product of the non-selective side reactions. A CFD integrated
multiscale method is developed for transport phenomena in both the solid and
fluid phases, for ethylene and n-butane oxidation reactions. CFD is coupled
with microkinetic models and aniotropic pellet diffusivity to study selectivity
and catalytic activity loss inside the reactor, and their connections to
elementary reaction steps, temperature, and catalyst structure. To date, no CFD
study has coupled detailed reaction kinetics inside the catalyst particles with
transport phenomena in the reactor. 2-Coupling microkinetics with CFD
Two different methods were developed to
couple microkinetic models with CFD. Initially, the proposed microkinetic
models were reduced to a single general reaction rate expression model. Later,
a method was developed and implemented to couple the full microkinetic model
with CFD.
The procedure of developing a general
rate expression for a microkinetic model is straightforward. The approach
includes several kinetic and mathematical assumptions to overcome the
complexity of the existing models. A reduced model for ethylene oxidation (EO)
based on a literature microkinetic model was obtained. Furthermore, literature
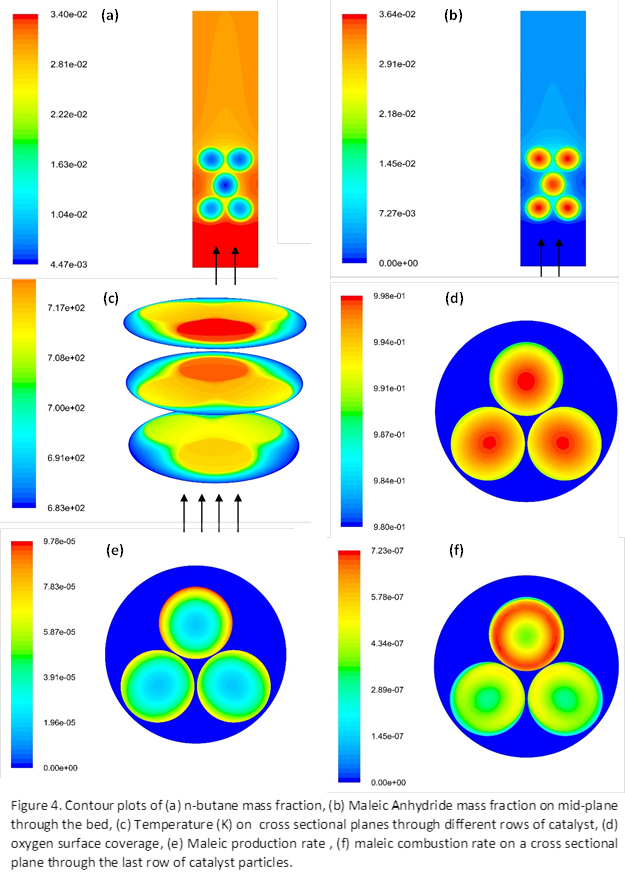
reduced models developed for n-butane oxidation were used for the maleic anhydride
reactor. To our knowledge these are the most comprehensive kinetic models existing
in the literature for the reactions of interest.
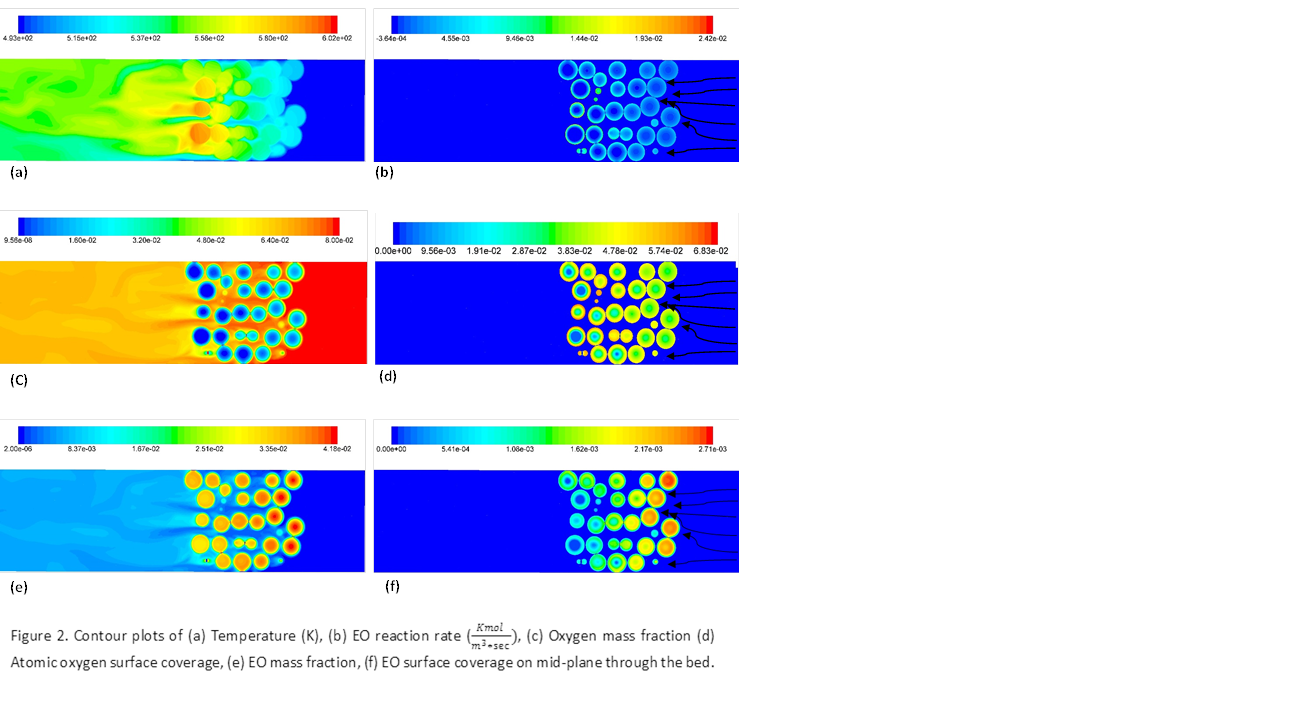
For the full microkinetic model of EO,
initially a sensitivity analysis was performed to evaluate the crucial
parameters to the model. Next, the model was solved over a wide range of
temperatures (T) and species partial pressures (Pi).
Reaction rates were then mapped into quadratic splines, and spline coefficients
were stored in a UDF as 1-D arrays. Finally, as CFD simulations were carried
out, for each computational cell, splines were constructed based on the
corresponding T and Pi to evaluate reaction rates.
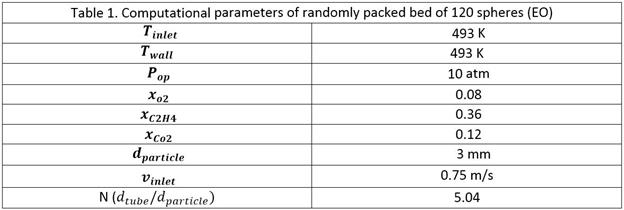
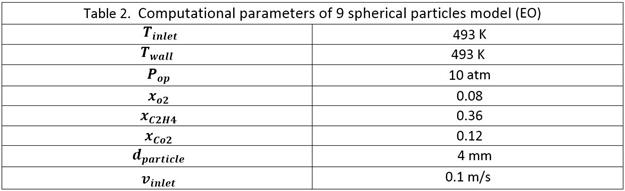
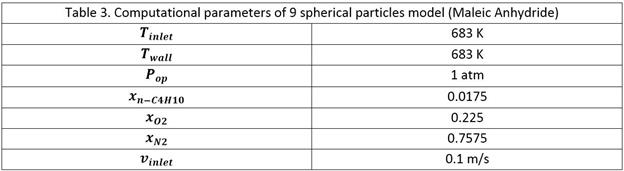
3-CFD simulations
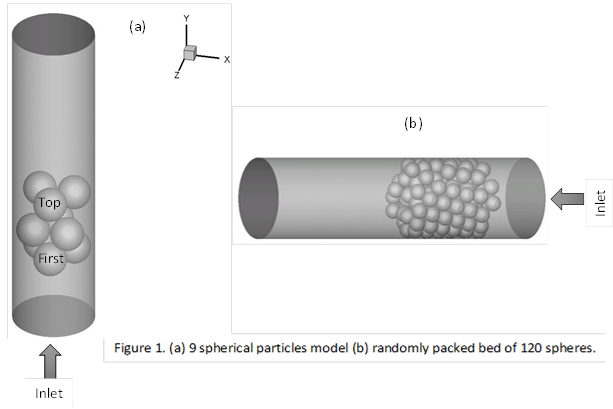
Two models are used for our studies
as shown in Figure-1.
3-1-Ethylene oxidation
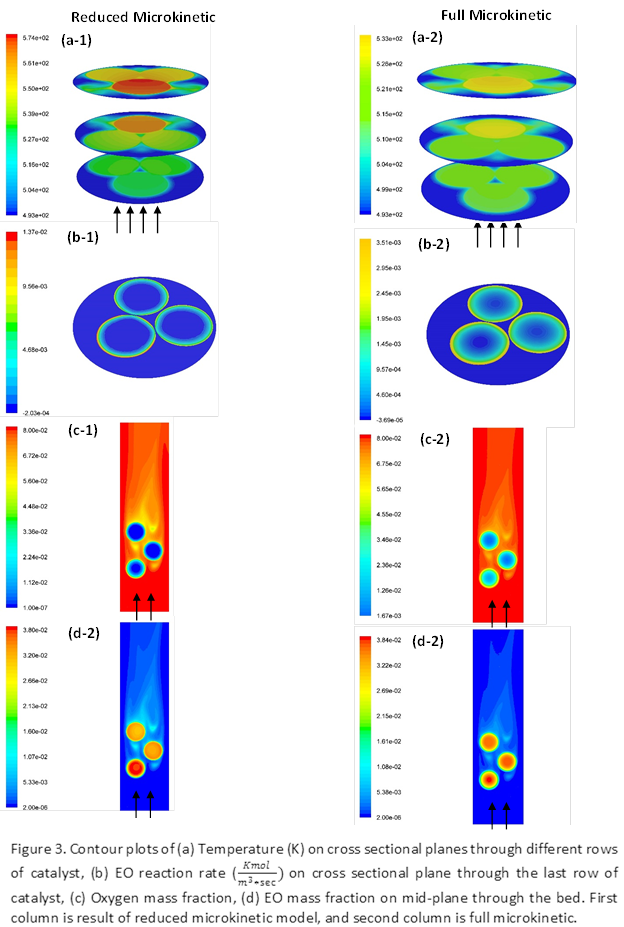
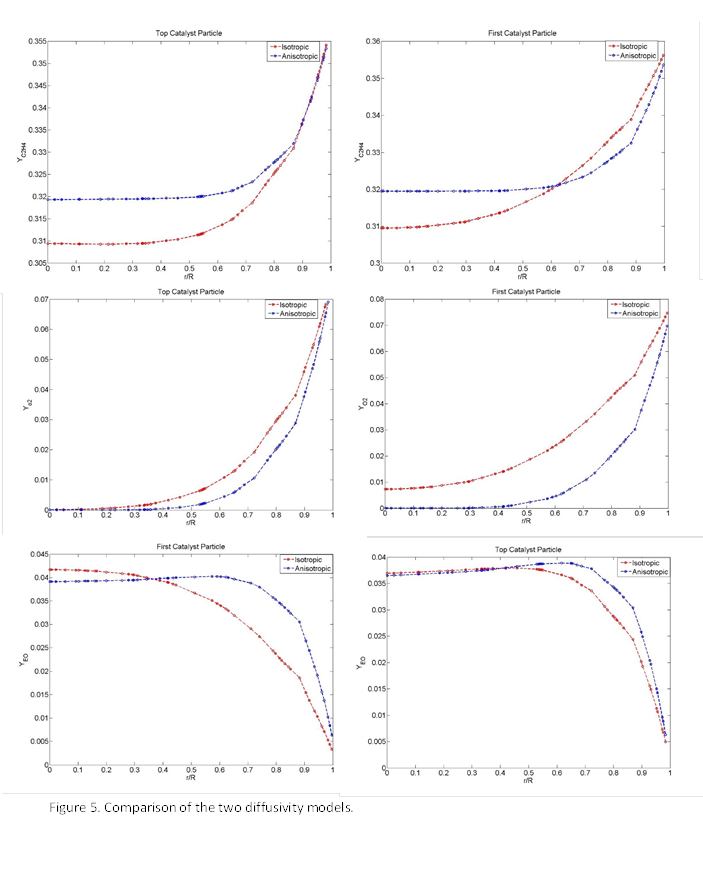
 4-Improving the Effective Pellet Diffusivity
4-Improving the Effective Pellet Diffusivity
| | 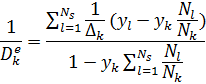 | |
| | 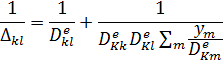 |
| | |