Reports: ND454262-ND4: Reaction Mechanism of C-H Activation of Fe-Catalysts
Junrong Zheng, Rice University
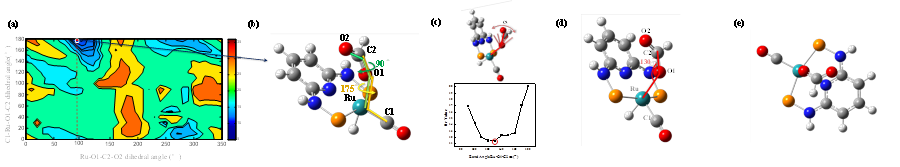
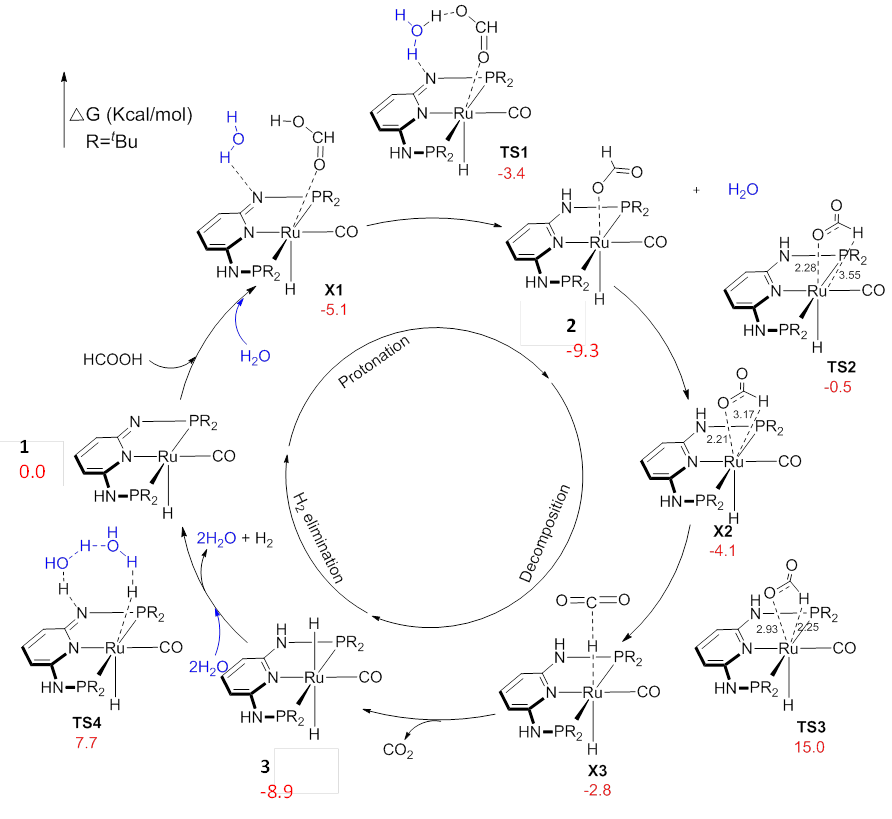
In the first fiscal year (09/2014-08/2015) supported by the ACS PRF grant PRF# 54262-ND4, my group achieved a significant accomplishment: for the first time, we demonstrated that the 3D structures of reaction intermediates which are too fast for NMR to measure can be determined by our vibrational cross angle method.
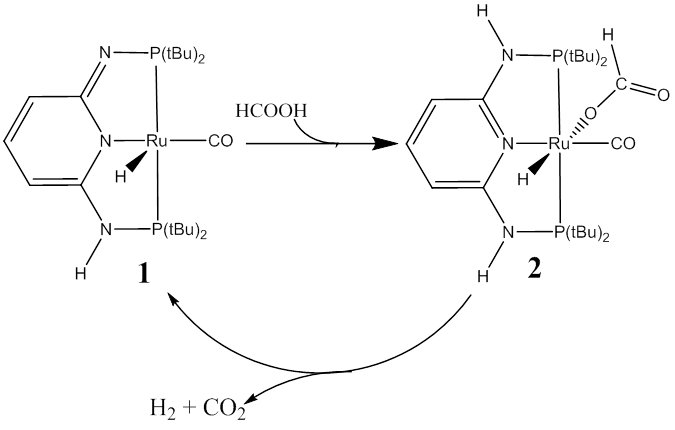
The reaction system investigated in this work
is the generation of hydrogen gas by decomposing formic acid, catalyzed by a
ruthenium PN3-pincer complex (
The decomposition of formic acid with the
presence of catalyst
This model system study allows us to explore all aspects of our method in determining reaction intermediate structures, which will be applied to the studies of other catalytic systems including the Fe-nonheme C-H activation catalysts in the near future.