Reports: DNI1053678-DNI10: New Strategies to Synthesize Covalent Organic Frameworks
Jian Zhang, University of Nebraska-Lincoln
The goal of this project was to develop new methods for the synthesis of covalent organic frameworks and porous organic frameworks. In the past year, we have identified a facile, environmental friendly strategy to synthesize carbazolic porous organic frameworks (Cz-POFs) and further explored their visible light photoredox catalytic activities.
1. Chemically tailorable synthesis of Cz-POFs with enhanced mesoporosity
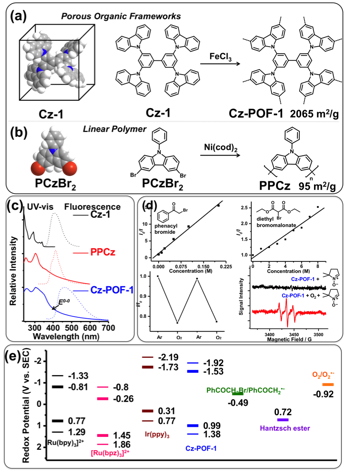
Carbazole is a fully ¹-conjugated aromatic molecule with excellent chemical stability. The nitrogen atom of carbazole can be easily substituted with a wide variety of functional groups to fine-tune its optical and electronic properties. Importantly, the 3- and 6-positions of carbazole are highly reactive, and upon oxidative polymerization using cheap, green oxidants such as FeCl3, poly(3,6-carbazole)s can be easily synthesized. We recently reported a facile synthesis of a series of carbazolic porous organic frameworks (Cz-POFs) using this method (Fig. 1a). We have shown that the mesoporosity of Cz-POFs can be significantly enhanced using dendritic monomers with high connectivity. For example, monomer Cz-1 with its four carbazole moieties can support up to eight cross-linking connections and the resulting Cz-POF-1 exhibits a remarkable Brunauer−Emmett−Teller (BET) specific surface area (SABET) (2065 m2/g−1). Importantly, 72% of the pore volume is contributed by mesopores (pore width > 2 nm), an ideal structural feature for organic reactions involving large-sized substrates or products. As a comparison, a linear polycarbazole, PPCz (poly- 9-phenyl-9H-carbazole, Fig. 1b), is essentially nonporous with an SABET of 95 m2/g−1.
Fig. 1. (a-b) Space-filling models of Cz-1 and PCzBr2 (3,6-dibromo-9-phenyl-9H-carbazole) (left) and their polymerization (right). (c) UV-vis and fluorescence spectra of Cz-1, PPCz, and Cz-POF-1. (d) Fluorescence quenching of Cz-POF-1 by phenacyl bromide, diethyl bromomalonate, oxygen (air), and ESR spectra of DMPO (black) and a mixture of DMPO and Cz-POF-1 (red) in aerated DMF. (e) Redox potentials of [Ru(bpy)3]2+, [Ru(bpz)3]2+, Ir(ppy)3, Cz-POF-1, and several substrates used.
2. Optical and electrochemical characterization of Cz-POF-1
Importantly, the extended ¹-conjugation in Cz-POFs derived from polymerization, albeit limited to a few repeat units, effectively decrease the HOMO–LUMO energy gap (Eg) and increase the visible light absorption cross-section. For instance, monomer Cz-1 has minimal visible light absorption; however, upon polymerization, the resulting Cz-POF-1 exhibits a broad absorption band that extends to the visible region (λ>390 nm) and shows an intense fluorescence band at 464 nm (Fig. 1c). In addition, linear polycarbazole PPCz also exhibits a broad absorption extending to the visible region (Fig. 1c), confirming that the extended ¹-conjugation indeed enhances visible light absorption. In combination with the cyclic voltammogram (CV) data, we calculated the E1/2 of Cz-POF-1+/Cz-POF-1 couple to be +1.38 V, and the E1/2 of Cz-POF-1+/*Cz-POF-1 couple was calculated as −1.53 V: both are stronger than those of *[Ru(bpy)3]2+. The strong reductive capability of *Cz-POF-1 was also supported by Stern−Volmer studies where the fluorescence of Cz-POF-1 can be efficiently quenched by electron deficient substrates such as phenacyl bromide (E1/2red = −0.49 V), diethyl bromomalonate, or molecular oxygen (E1/2red = −0.92 V) (Fig. 1d). In particular, the reduction of oxygen by *Cz-POF-1 generates reactive species superoxide anion (O2¥−), which was revealed by electron paramagnetic resonance (EPR) spectroscopy: strong EPR signals were observed when DMPO (5,5-dimethyl-1-pyrroline N-oxide), a radical trapping agent of O2¥−, was present in an aerated DMF solution of Cz-POF-1 upon light irradiation (Fig. 1d).
3. Visible light photoredox catalytic activities of Cz-POF-1
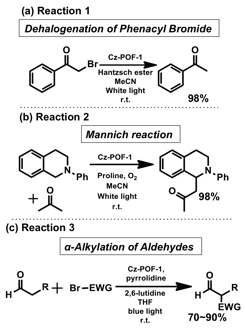
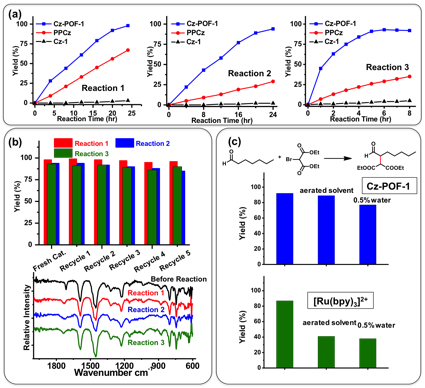
With the knowledge of its photoredox potentials in hand, we demonstrated the its applications in organic transformations using net reductive dehalogenation of phenacyle bromide (Reaction 1), net oxidative Mannich reaction (Reaction 2), and redox neutral α-alkylation of aldehydes (Reaction 3) (Scheme 1). In each case, we found Cz-POF-1 underwent efficient oxidative quenching pathways and exhibited remarkable photoredox catalytic activities and wide substrate scope. More importantly, several other attributes of Cz-POF-1 were noteworthy. First, the unique extended ¹-conjugation leads to the enhanced visible light absorption and large mesoporosity of Cz-POF-1 enables exceptional reaction rate. For example, monomer Cz-1 essentially gave no conversion and nonporous linear polymer PPCz required a longer reaction time and led to poor yield for all three reactions (Fig. 2a). This property also differentiate Cz-POF-1 from other approach, which heterogenization of metal complexes and organic dyes in POFs were used for several organic transformations since the photoredox activities of Cz-POFs are due to the tunable optoelectronic properties of the three-dimensional ¹-conjugated polymeric structures, and not due to chromophore immobilization. Second, Cz-POF-1 can be easily recovered and reused. Neither significant decrease of reactivity nor structural change was observed after reusing Cz-POF-1 (Fig. 2b), suggesting a satisfactory stability. Moreover, a 2.5 mmol scale trial led to an excellent isolated yield (87%), indicating the feasibility of using Cz-POF-1 for scale-up synthesis. Third, reactions catalyzed by Cz-POF-1 require less strict conditions. For instance, oxygen and moisture residue (0.5% water) in the aerated solvent significantly decreased the yield and selectivity for the reaction between heptaldehyde and diethyl bromomalonate when [Ru(bpy)3]2+ was used as the catalyst (Fig. 2c). However, such detrimental effect was insignificant for reactions catalyzed by Cz-POF-1, possibly due to the hydrophobic pore environment.
Scheme. 1
Fig. 2. (a) Comparison of rates of conversation using Cz-POF-1, PPCz, and Cz-1 as the photocatalyst. (b) Recyclability and (c) stability test of Cz-POF-1. (d) Reaction conversion comparison between Cz-POF-1 and [Ru(bpy)3]2+ for Reaction 3 using solvents with different content of oxygen and moisture.
Summary.
Funding for this project has allowed us to not only explore new synthetic strategies for preparation of functional porous organic frameworks, but also their novel applications in photoredox catalysis for organic transformations. The Cz-POFs based heterogeneous solid offer a new class of solid photocatalysts that can be recycled and reused. The PRF fund has allowed my research group to collect sufficient preliminary results for applying for other funding agencies.