Reports: UR452071-UR4: Hydrogen-Bonding Control of Solvatochromism and Non-Radiative Decay in the Fluorescence of PRODAN Derivatives
Christopher J. Abelt, PhD, College of William and Mary
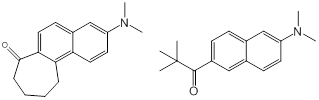
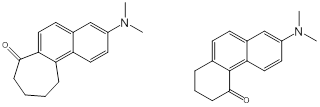
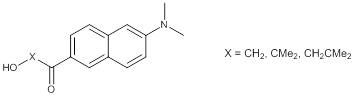
Christopher J. Abelt, PhD, College of William and Mary



Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society