Reports: UR752090-UR7: Novel Polymer Coupling via Thiazolidine Chemistry
Philip J. Costanzo, PhD, California Polytechnic State University
Thiazolidine chemistry is a commonly used reaction used in biological systems because the reaction requires the presence of both cysteine (a common amino acid) and an aldehyde or ketone. The cysteine residue contains multiple functionalities that be employed to prepare unique materials. We have developed a synthetic route to access three different functional points of the cysteine residue. Futhermore, protected cysteine molecules have been successfully ligated onto polymerizable monomers and have been shown to be easily deprotected in the presence of an acid source.
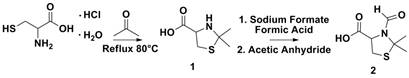
Scheme 1 Synthesis of protected cysteine.
The synthesis of 1 was simple and efficient. Various protecting groups were employed with a wide range of synthetic conditions. Ultimately, a mixed anhydride method was employed. The synthesis of 2 was confirmed directly via 1H NMR after it was recrystallized from methanol and water.
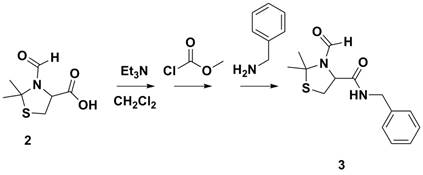
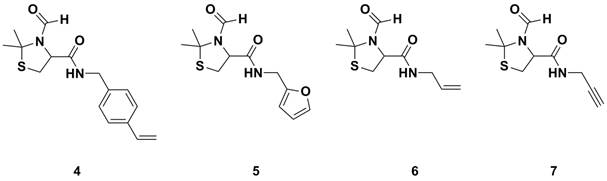
Next, the carboxylic acid functional group was coupling with various amines. Scheme 2 depicts coupling with benzyl amine which was conducted as a control experiment. Other amines, such as allyl amine and propargyl amine have employed to allow for thiol-ene/yne chemistry, furfuryl amine to allow for Diels-Alder chemistry and 4-vinyl benzyl amine for polymerizable monomers, Figure 1.
Scheme 2. Carboxylic acid coupling of protected cysteine.
Figure 1. Functional protected cysteine compounds prepared.
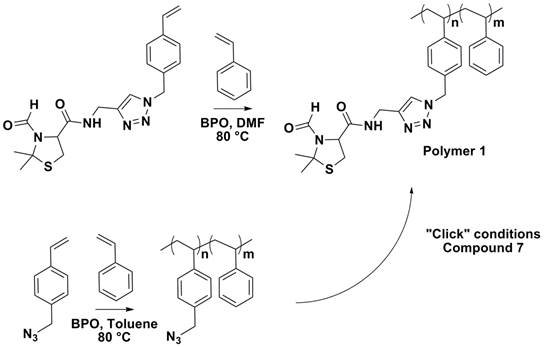
Protected cysteine residues were incorporated into polymeric resins by the direct polymerization of compound 4, as well as the post-polymerization modification of poly(styrene-co-4-vinyl benzyl azide), Scheme 3.
Scheme 3. Incorporation of protected cysteine into polymeric systems
Polymer 1 can be treated under acidic conditions to expose the cysteine functionality, which can then undergo thiazolidine coupling. Organo gels are currently being prepared an evaluated. Additionally, thermo-responsive polymers such as poly (N-ispropyl acrylamide) are being synthesized to be utilized in the preparation of hydrogels.
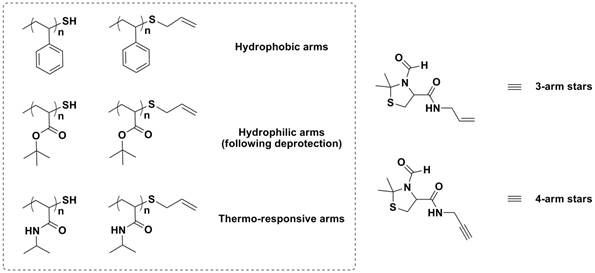
Finally, compounds 6 and 7 have been utilized as cores for the preparation of miko-arm star polymers via thiol-ene/yne coupling, Scheme 4. Using thiol-ene/yne chemistry arms of different composition and philicity can be attached in sequential order. Followed by carbonyl or isocyanate coupling to add the final arm. Either three or four armed stars can be prepared by utilizing either compound 6 or 7, respectively. We are currently in the last steps of synthesis for the three and four armed stars. Future work will explore the solution state properties of the compounds.
Scheme 4. Components for miktoarm stars based upon cysteine cores
In total, seven undergraduate students have completed research from support of this award and resulted in nine presentations given by undergraduate students at the National American Chemical Society meetings, including 6 oral presentations and 3 poster presentations.