Reports: ND1053490-ND10: Metal-Organic Framework Catalyst for Organic Compound Conversion
Nianqiang Wu, PhD, West Virginia University
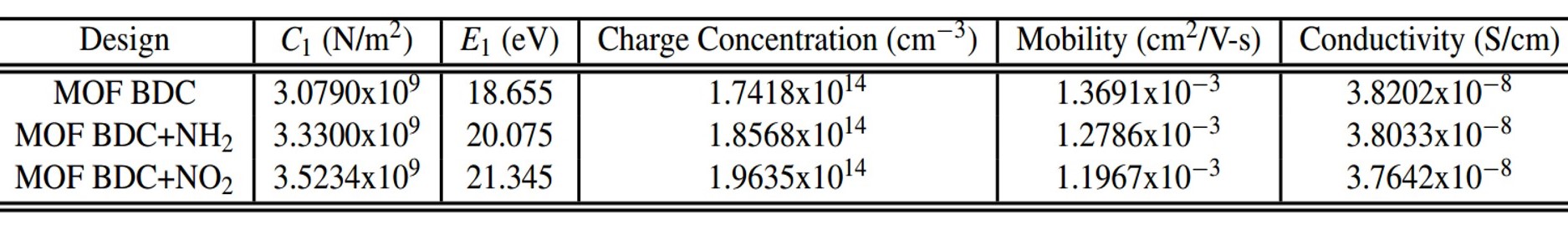
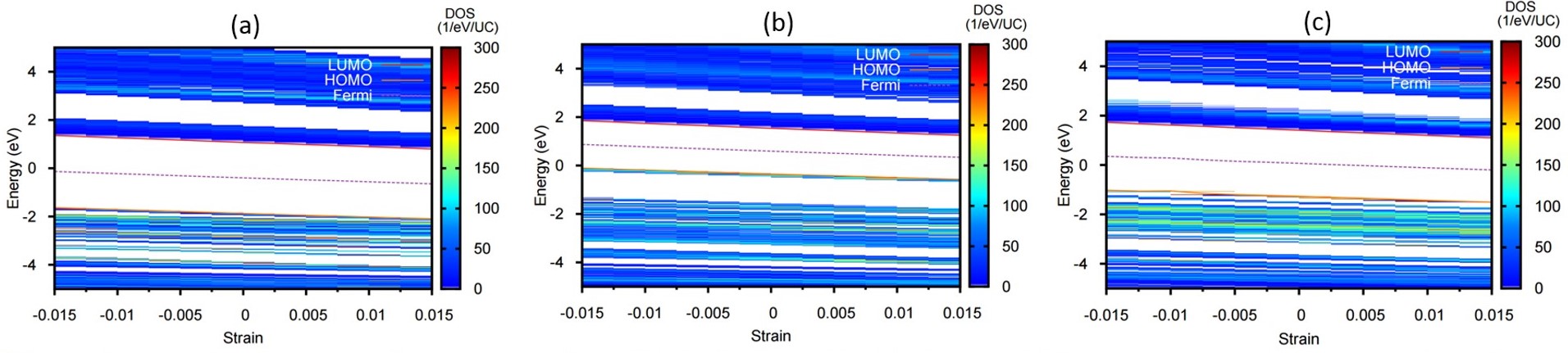
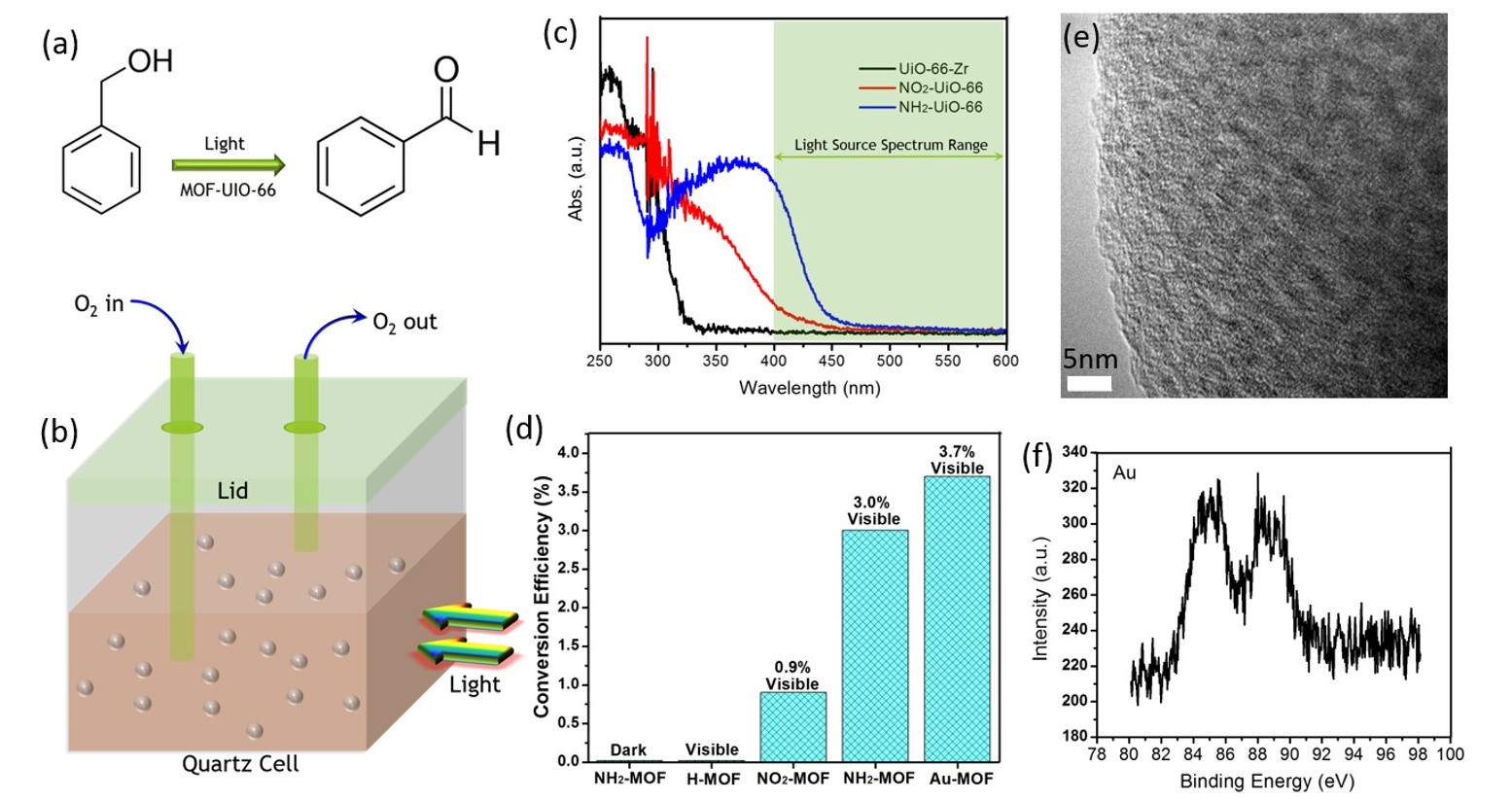
Nianqiang Wu, PhD, West Virginia University



Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society