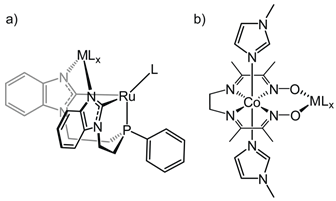
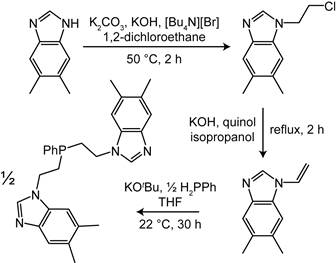
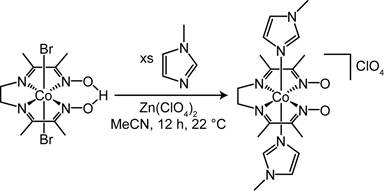
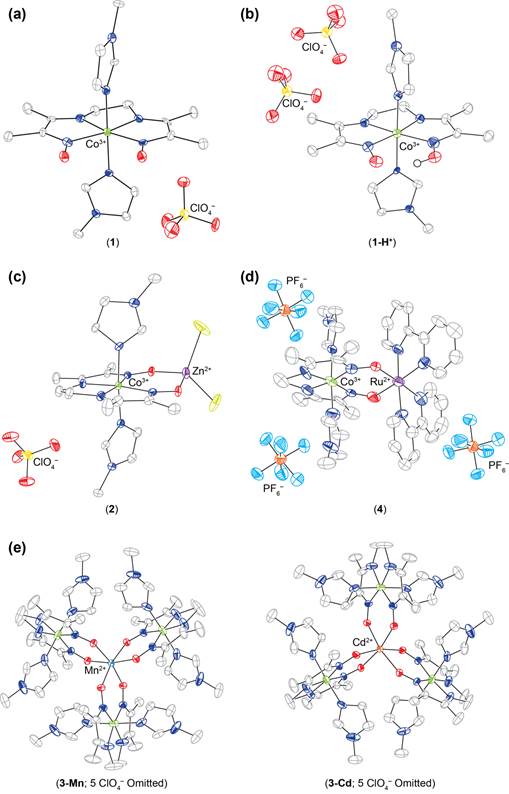
Reports: DNI354226-DNI3: Homogeneous Bimetallic Systems for Syngas Conversion
Brandi Cossairt, University of Washington
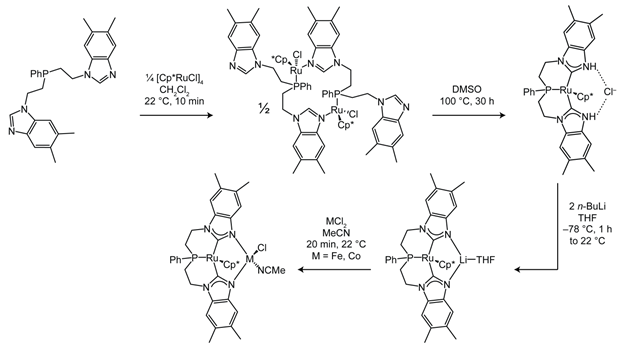
Scheme 3.
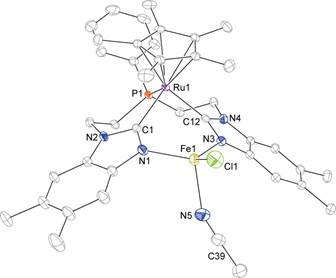
Synthesis of cis-doubly deprotonated diimine dioximate metalloligand. Figure 3.
Single crystal X-ray diffraction structures of mono- bi- and tetrametallic
complexes containing the cobalt diimine dioximate metalloligand.