Reports: ND653264-ND6: Thermodynamic Insights into Surfactant Assembly in Protic Ionic Liquids
Henry Ashbaugh, PhD, Tulane University
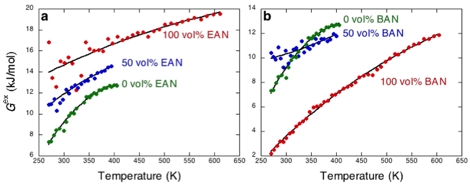
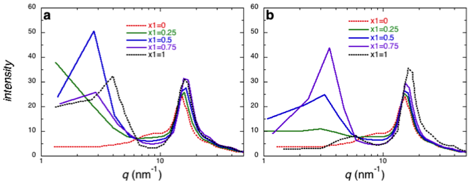
Henry Ashbaugh, PhD, Tulane University


Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society