Reports: DNI753195-DNI7: Synthesis of Multivalent and Multimodal Nanoparticles by 'Clicking-To' Multiblock Dendrimers
Jonathan G. Rudick, PhD, State University of New York at Stony Brook
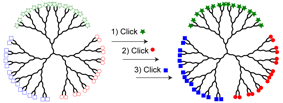
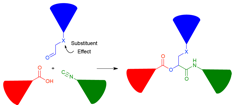
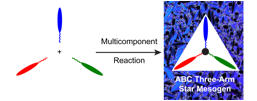
Jonathan G. Rudick, PhD, State University of New York at Stony Brook



Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society