Reports: ND754177-ND7: New Catalysts for Stereoselective Polymerization of Functional Alpha-Olefins
Lin Pu, University of Virginia
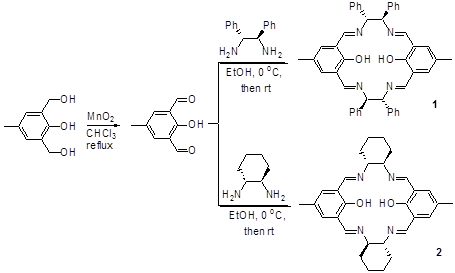


Lin Pu, University of Virginia



Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society