Reports: UR252201-UR2: Speciation and Sequestration of Rhenium in Sulfidic and Polysulfidic Natural Waters
Trent Vorlicek, PhD, Minnesota State University Mankato
Project Objectives: The overarching purpose of this project is to define better the chemical pathway leading to Re deposition within anoxic waters. Recent evidence from the Black Sea suggests that Re removal may be linked to Mo deposition via co-precipitation of Re with a posited FeMoS solid phase (i.e., Fe5Mo3S14). This potential relationship has led the project to include batch experiments involving formation of FeMoS solid(s) in the presence of trace concentrations of ReO4-. These experiments aim to determine thermodynamic constants for the FeMoS solid(s) as well as quantify any fractionation occurring between Re and Mo during their possible co-precipitation. The project also seeks to develop a reverse phase ion pair chromatography (RP-IPC) method for separating mixtures of all oxythiomolybdates (MoO42-, MoO3S2-, MoO2S22-, MoOS32-, and MoS42-) and all oxythioperrhenates (ReO4-, ReO3S-, and ReS4-; ReO2S2- and ReOS3- are kinetically unstable.). Eventual coupling of RP-IPC to ICP-MS may allow for simultaneously quantifying actual Mo and Re speciation within natural sulfidic waters. Because Mo and Re have stable isotopes, RP-IPC with MS may be used in the future to quantify isotopic fractionation occurring as MoO42- or ReO4- transforms into MoS42- or ReS4-.
Research Progress:
a.) Co-precipitation of Re with an FeMoS solid phase
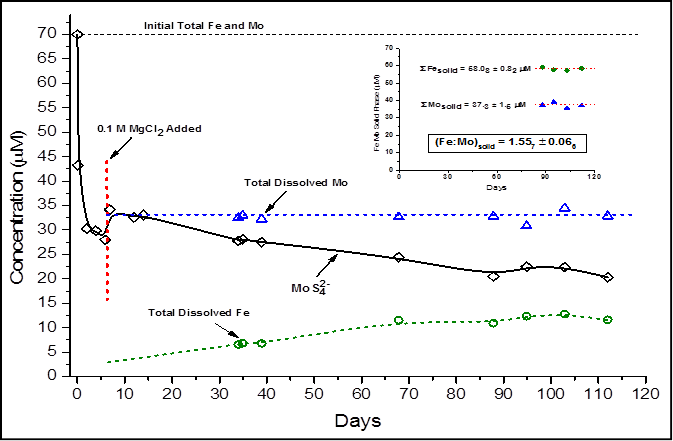
Figure 1 shows the reaction progress upon addition of 70 mM MoS42- to a solution containing initially equimolar Fe2+ and 10 mM SS2- at pH = 8.5. At ~6 days, 0.1 M MgCl2 was added to the reaction mixture to cause flocculation of initially formed colloids that would otherwise escape filtering. The main graph in the figure displays an initial rapid loss of MoS42- followed by a sluggish decay until equilibrium is achieved after ~85 days. Concomitantly, total dissolved Fe begins to accumulate in solution until it appears to reach equilibrium with MoS42-. Total dissolved Mo remains constant upon MgCl2 addition. These results imply the formation of FeMoS solid and aqueous phases. In the aqueous phase, the stoichiometry of the FeMoS complex appears to be 1:1 in Fe:Mo, consistent with 1:1 FeMoS aqueous complexes (e.g., [(Fe2S2)(MoS4)2]4-) known to form in the presence of pyrrhotite surfaces.
The inset in the figure shows data related to the FeMoS solid phase. The y-axis in the inset is calculated from the difference between initial total Mo and Fe and equilibrium values of dissolved Mo and Fe. Total Fe and total Mo associated with the FeMoS solid yield an Fe:Mo ratio of 1.56 ± 0.07. This ratio is in very good agreement with that of Fe5Mo3S14 (Fe:Mo = 1.7 ± 0.7), the solid posited to account for Mo removal (and possibly Re) in euxinic water columns.
Figure 1: Reaction progress upon addition of 70 mM MoS42- to a solution containing equimolar Fe2+ and 10 mM SS2- at pH = 8.5. Although the solution is saturated WRT FeSmackinawite, the system becomes dominated by FeMoS(aq,s) chemistry at equlibrium (~85 days). If mackinawite controlled Fe solubility, SFedissolved ~ 0.1 nM. Note that FeMoS(aq) is ~1:1 Fe:Mo at equilibrium (Mo in FeMoS(aq) = dashed blue minus solid black line). FeMoS(s) equilibrium data shown in inset graph. Total metal concentrations quantified by AAS; MoS42- quantified by UV-vis.
The preliminary work shown in Figure 1 played an important role in helping to secure a NSF EAGER grant for my colleague, Anthony Chappaz (Central Michigan University), and me. Dr. Chappaz and I have collaborated on trace metal geochemistry projects over the past several years. The NSF EAGER grant provides funding through March 2016.
While the Fe-Mo-S work appears quite promising, we encountered difficulties over the past year in quantifying Total Mo concentrations using AA or ICP-MS. Measurements yield systematically high (i.e., greater than initial values) and erratic concentrations of Total Mo. Previous work has cited similar difficulties measuring Total Mo in Fe-Mo-S containing solutions. Unfortunately, much time has been spent attempting to determine the cause of the systematic error. However, it appears we have identified ionic strength as the likely culprit. In order to improve the accuracy of activity coefficient calculations necessary for obtaining accurate equilibrium constants, we decided to lower the ionic strength by reducing MgCl2 to 0.01 M in new batch experiments from the 0.1 M MgCl2 indicated for the test solution in Fig. 1. The new MgCl2 concentration was chosen because experiments appeared to show the critical concentration of MgCl2 for FeMoS flocculation was just over 0.001 M. However, optical data of the filtered (0.45 mm) batch solutions over time indicates an increasing presence of colloids which escape filtering. Colloids could explain the systematic errors. Presently, we are preparing new batch experiments at 0.1 M MgCl2. Assuming useful results from these new experiments, our aim is to prepare a manuscript for submission by the end of 2015.
b.) Separation of all oxythiomolybdate and all oxythioperrhenate anions
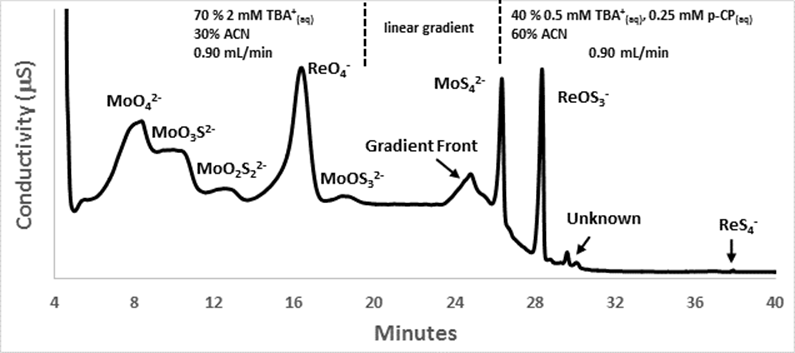
Figure 2 displays a chromatogram demonstrating the successful separation of a mixture containing all eight oxythiomolybdate and oxythioperrhenate anions at mM concentration. These data as well as separations for oxythiomolybdates and oxythioperrhenates were published in Chemical Geology* in 2015. This separation technique will assist future work seeking to quantify Mo and Re speciation in natural waters as well as any isotopic fractionation occurring during transition of these metals to thiometalates.
Figure 2: Chromatogram showing separation of all stable oxythiomolybdates and oxythioperrhenate anions at mM concentrations using RP-IPC and conductivity detection. TBA+ = tetrabutylammonium cation; ACN = acetonitrile; p-CP = p-cyanophenol (added to promote elution of strongly retained MoS42-, ReOS3-, and ReS4-).
Student participation, publications, and presentations: One publication in Chemical Geology* and two presentations were supported by PRF during 2014-2015. A total of four undergraduates participated in the project over the past year. Two students presented their results at the Spring 2015 ACS national meeting.
*Vorlicek, Trent P., Chappaz, Anthony, Groskreutz, Laura M., Young, Natalie, and Lyons, Timothy W. (2015) A new analytical approach to determining Mo and Re speciation in sulfidic waters. Chem. Geol. 403, 52-57.