Reports: UNI152706-UNI1: Catalytic Methylation of Oxygen Nucleophiles with Safe, Stable Methylating Agents
David J. Gorin, PhD, Smith College
Overview of Research Program:
Methylation reactions of carboxylic acids and other nucleophiles are widely used in chemical research, including in natural product synthesis, medicinal chemistry, methods development, and polymer chemistry. Although often effective, Fisher esterification is incompatible with acid-sensitive substrates. This led to the development of electrophilic methylating agents that react under mild conditions, such as diazomethane and dimethyl sulfate. Unfortunately, commonly-used electrophilic methylating agents are hazardous, unstable, and/or toxic. For example, two laboratory-related deaths have been attributed to trimethylsilyl diazomethane, which was developed as a safer, less-explosive alternative to diazomethane.
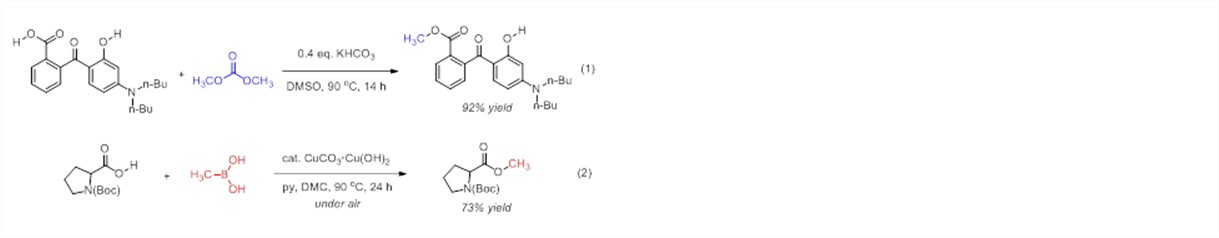
Inspired by others' successes in developing non-explosive carbene precursors and safer alternatives to hydrogen cyanide, we have initiated a program to develop new methylation methods for oxygen nucleophiles that rely upon safe, stable reagents. Increased safety and convenience will reduce the cost and risk of a ubiquitous transformation. Specifically, our laboratory has explored two alternate strategies for O-methylation. In the first, dimethyl carbonate was used as a non-toxic and "green" electrophilic reagent, and a base-catalyzed, chemoselective methyl transfer from dimethyl carbonate to carboxylic acids was developed (eq 1).1 In the second strategy, a nucleophilic methylating reagent was used in place of typical electrophilic methyl sources. Specifically, the Cu-catalyzed oxidative coupling of methylboronic acid and carboxylic acids to synthesize methyl esters was discovered and developed (eq 2).2
Current Results:
In the current report period, our most significant results are in the discovery and development of an aerobic Cu-catalyzed O-methylation using methylboronic acid.2 In this work, a nucleophilic methyl source is used, in contrast to commonly-used electrophilic reagents. One advantage of this strategy is that it avoids the intrinsic toxicity associated with electrophiles. Furthermore, coupling of nucleophilic agents with oxygen nucleophiles requires umpolung reactivity and the use of some oxidant, necessitating fundamentally different reaction pathways than those accessed in traditional methylation reactions. This may enable reaction selectivity and functional group tolerance that complement other methods.
Methylboronic acid, a commercially-available white solid easily handled in the laboratory, was identified as a promising nucleophilic reagent for O-methylation. The Cu-catalyzed oxidative coupling of arylboronic acids and heteroatom nucleophiles (Chan-Lam reaction) is well-developed and seemed a promising starting point for reaction discovery, although Chan-Lam alkylation with alkylboronic acids is rare. Furthermore, there are few examples of Chan-Lam arylations with carboxylic acids, perhaps because these substrates are prone to decarboxylation. Nevertheless, encouraged by these precedents, we investigated the Cu-catalyzed synthesis of methyl esters by cross-coupling of methylboronic acids and carboxylic acids.
Optimized reaction conditions for the esterification of carboxylic acids with methylboronic acid were determined by screening copper sources, basic additives, and solvents. The combination of cupric carbonate (10 mol%) and pyridine in dimethyl carbonate under air provided methyl esters in good yields (eq 2). Although dimethyl carbonate may serve as a methylating reagent,1 it does not do so under these conditions, as demonstrated by the efficacy of the reaction using diethyl carbonate as the solvent and the reaction’s failure upon omission of methylboronic acid. The importance of oxygen in air as the terminal oxidant was confirmed by investigating the reaction under a nitrogen atmosphere, where no product was formed, even upon addition of water. Notably, these conditions represent a rare use of catalytic copper under aerobic conditions for Chan-Lam alkylation.
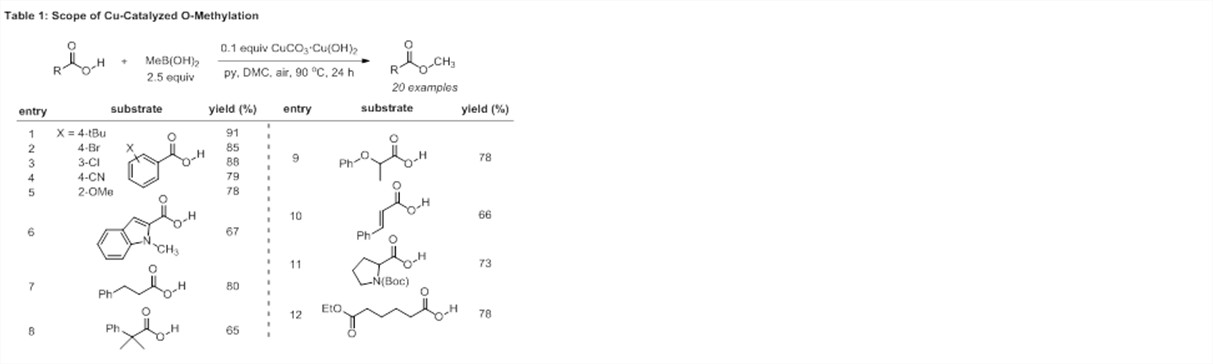
To determine the scope of the reaction, the optimized reaction conditions were applied in the methylation of aryl, alkyl, and alkenyl carboxylic acids (Table 1).2 Benzoic acid derivatives bearing both electron-donating and electron-withdrawing substituents were smoothly transformed into the corresponding methyl esters (entries 1-5). Sterically hindered substrates, including a neopentylic carboxylic acid, were tolerated (entries 5, 8). Substrates sensitive to strongly acidic (entry 11) or strongly basic (entries 9, 12) conditions were also methylated with methylboronic acid, demonstrating that the reaction conditions are relatively mild.
Mechanistic studies support a Chan-Lam-type cross-coupling mechanism between methylboronic acid and the carboxylic acid (Scheme 1, path A).2 Evidence against a Fischer esterification mechanism proceeding by in situ oxidation of methylboronic acid to methanol (Path B) was accumulated through two different experiments. First, no product was observed upon replacement of methylboronic acid with methanol. Second, an isotope-labeling study revealed that both oxygen atoms present in the starting carboxylic acid were retained in the methyl ester product. Both of these results are consistent with path A and inconsistent with path B, suggesting that a cross-coupling mechanism is operative.
Impact:
The methylation project has enabled an array of opportunities for undergraduate researchers to conduct and present their work. Seven students have worked on the project during the grant period. Five are co-authors on the two J. Org. Chem. publications that resulted from the work described above. Three students completed honors theses, and three others will do so in the coming year. Support from this grant funded six full-time summer undergraduate research positions. Furthermore, PRF funding enabled three students to present a poster at the March 2014 national ACS conference. Other students presented at the 2015 National Organic Symposium, as well as regional and campus scientific conferences. Finally, PRF funding provided resources for my lab to pursue two separate project areas, and the second (non-methylation) project created research and presentation opportunities for six additional undergraduate students.
[1] "Catalytic Methyl Transfer from Dimethylcarbonate to Carboxylic Acids," Ji, Y.; Sweeney, J.; Zoglio, J.; Gorin, D. J. Journal of Organic Chemistry 2013, 78, 11606-11611.
[2] "Aerobic Copper-Catalyzed O-Methylation with Methylboronic Acid" Jacobson, C. E.; Martinez-Munoz, N.; Gorin, D. J. Journal of Organic Chemistry 2015, 80, 7305–7310.