Reports: DNI1053874-DNI10: Enhancing Oxidation Catalysis with Ti-Based Metal Organic Frameworks by Tuning Structure and Composition
Jason Hicks, PhD, University of Notre Dame
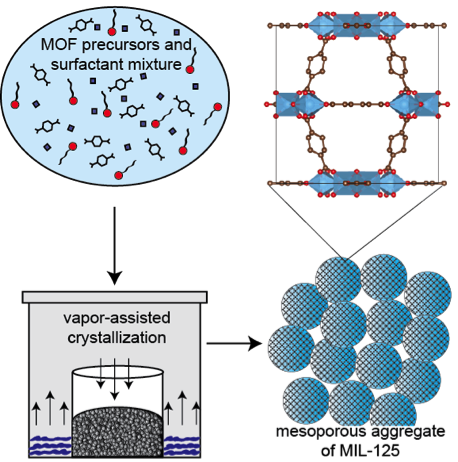
Prior to this awarded grant, we explored the use of two MOFs, (V) MIL-47 and (Ti) MIL-125, as heterogeneous catalysts for the oxidation of thiophenic compounds. We found that while the V-based MIL-47 displayed high activity, it was unstable under reaction conditions. The Ti-based MIL-125, however, although not as active as MIL-47, was stable even under harsh conditions. The lower activity of MIL-125 was attributed in part to its small pore size that could not accommodate bulky thiophenic complexes such as dibenzothiophene (DBT). Therefore, we proposed to synthesize a mesoporous analogue of MIL-125 (this ACS PRF grant). We accomplished this task through the use of a synthetic procedure (vapor-assisted crystallization, VAC, Figure 1) that was inspired by our previous experience with zeolite synthesis. In this method, a dried precursor powder containing MOF precursors and an ionic surfactant templating agent (cetyltrimethylammonium bromide) were subjected to VAC to facilitate the formation of hierarchically mesoporous/microporous MIL-125 similar to steam-assisted crystallization of zeolites.
Figure 1. Formation of hierarchically microporous and mesoporous
MIL-125 using the vapor-assisted crystallization method developed in the Hicks
Lab. The newly synthesized mesoporous
MIL-125 (meso-MIL-125) was fully characterized using a number of techniques. N2
physisorption analysis was used to determine the
meso-MIL-125 contained significant amounts of both microporosity
and mesoporosity while XRD diffraction patterns
showed the new material was composed of crystalline MOF. The meso-MIL-125
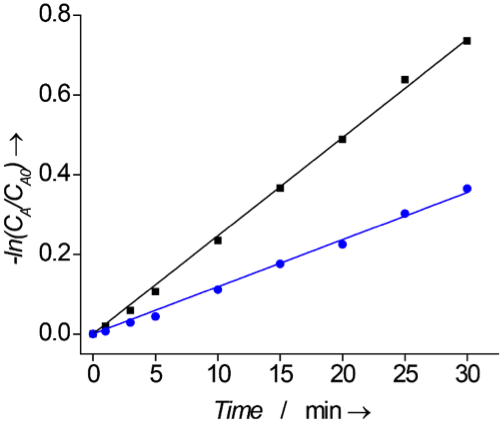
material was then tested as a catalyst in the oxidation of DBT by