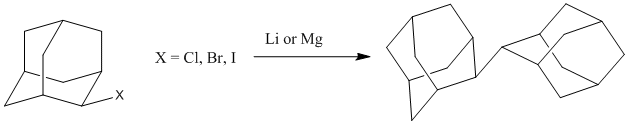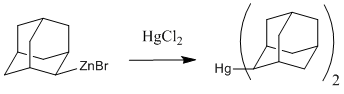Reports: ND354167-ND3: Metals at Tripodal Sites of Adamantane and Graphane-Like Surfaces of Nano-Diamonds: A Unified Strategy for Petroleum-Derived Materials from the Molecular to the Nano-Scale
Ulrich Fekl, University of Toronto
We are working on synthesizing previously inaccessible adamantane derivatives, including higher polymantanes, via C-H bond activation of adjacent-to-the-metal axial C-H bonds in 2-adamantyl transition metal complexes. This approach will need access to adamantanes metallated (with a transition metal) in the bridge position, not the more easily accessible bridgehead position. However, there are currently no transition metal 2-adamantyls known, the only exception being metal-coordinated 2-adamantyl fragments as part of a larger ligand (pincer ligand). Therefore, we have been exploring the chemistry of the 2-adamantyl anion.
The synthesis of simple anionic 2-adamantyl precursors such as alkyl lithium or Grignard reagents is not straightforward, quite in contrast to the situation for other alkyls. Synthetic methods to prepare 2-adamantyl lithium are absent from the literature. While the synthesis of the 2-adamantyl Grignard reagent has been reported [1], the reaction is extremely unreliable, and a later publication acknowledged the strange fact that the outcome of the reaction is very sensitive to the stirring speed of the solution [2]. In our hands, the reaction of 2-bromoadamantane (2-AdBr) with lithium or magnesium resulted in the formation of coupling products (primarily 2,2’-biadamantyl) (Figure 1) with only small amounts of the metal adamantyl observable in solution.
Figure 1.
Two routes have proven to be useful however, both through the use of Rieke-type activated metals. 2-Adamantylzinc bromide (2-AdZnBr) can be synthesized reliably from 2-AdBr and Rieke zinc. Alternatively, we have experimental evidence to suggest that a 2-adamantyl calcium species can be produced by using Rieke calcium. The two anionic species have vastly different reactivities. Synthesizing 2-AdZnBr requires refluxing in tetrahydrofuran for several days, while the formation of adamantyl calcium proceeds within minutes at -90 ⁰C. While both compounds open up possibilities for transmetallations onto other metals, both have their own challenges. The adamantyl calcium complex is extremely reactive and decomposes just above -50 ⁰C. On the other hand, 2-AdZnBr is unreactive and does not seem to easily transfer 2-adamantyl on other metals, in particular early transition metals. However, we found that both compounds can be used to transmetallate the 2-adamantyl group onto trialkyl- or triarylphosphine gold(I) chloride, producing R3PAu(2-adamantyl) compounds (R = phenyl and cyclohexyl). Since 2-AdZnBr does not appear to transmetallate onto early metals, a different approach is needed in order to synthesize the desired early metal 2-adamantyl complexes. We are exploring reductive routes, in which a highly reducing metal (such as lithium, sodium, or magnesium) is used to reduce the central metal of the alkyl compound, which contains are more oxidizing metal. Activated magnesium turnings were successfully used to reduce 2-AdZnBr to zinc powder and produce 2-adamantylmagnesium bromide (2-AdMgBr).We have thus successfully developed an indirect but reliable procedure for synthesizing 2-adamantyl magnesium bromide. We speculate that a similar procedure could developed in which an activated metal powder of an early transition metal (e. g., Rieke yttrium, for example) could be used to synthesize the early metal 2-adamantyl from the zinc precursor. Curiously, the reaction of Rieke yttrium and 2-AdZnBr cleanly produces bis(2-adamantyl)zinc only. Bis(2-adamantyl)zinc forms with 2,2’-bipyridyl a red complex, similarly to other zinc dialkyls. Since Rieke yttrium appeared to not be sufficiently reducing to reduce zinc(II) in adamantyl zinc bromide, we focused on adamantyl mercury complexes. Bis(2-adamantyl)mercury was successfully synthesized from 2-AdZnBr and HgCl2 (Figure 2). We are currently working on reductive transmetallation using the newly discovered bis(2-adamantyl)mercury.
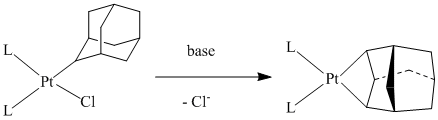
We also have NMR-spectroscopic evidence for 2-adamantyl platinum complexes, made from (1,5-COD)PtCl2 and 2-AdMgBr, which should offer the potential for CH activation as shown in Figure 3.
Figure 3.
Our intermediate results have extended the extremely limited chemistry of the 2-adamantyl anion. We have shown that 2-adamantyl zinc bromide, which is straightforwardly synthesized, can be used to generate the hard-to-obtain 2-adamantyl magnesium bromide. Furthermore, we have synthesized the first 2-adamantyl complexes of mercury and gold and we have spectroscopic evidence for a platinum complex. We are working on CH activation of axial CH bonds with the 2-adamantyl species.
This PRF grant has supported one graduate student (David Armstrong) since 2014, who obtained most of the results described here. A second graduate student (Fioralba Taullaj) joined the project in fall 2015. One or two undergraduate students are typically involved in this project as well.
References
[1] Dubois, J.-E.; Bauer, P.; Molle, G.; Daza, J. C. R. Acad. Sci., Ser. C.1977, 284, 145-148.
[2] G. Molle, P. Bauer, J.E. Dubois, J. Org. Chem. 1982, 47, 4120.